15th February 2013 FDA approves first bionic eye in the US After more than 20 years of research and development, Second Sight Medical Products has been granted market approval from the Food and Drug Administration (FDA) for its Argus II Retinal Prosthesis System.
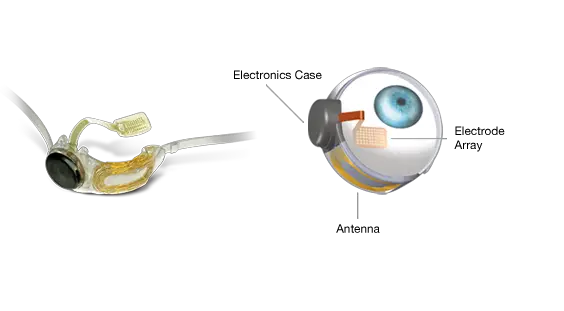
The Argus II is the first implanted device to treat advanced retinitis pigmentosa (RP) – a degenerative disease that often results in nearly complete blindness, and affects roughly 100,000 Americans. The device features a small video camera, transmitter mounted on a pair of eyeglasses, video processing unit (VPU) and a retinal prosthesis (artificial retina). Together, these can replace the function of degenerated cells in the retina (a membrane inside the eye) to improve a patient's ability to perceive images and movement. The VPU transforms images from the video camera into electronic data that is wirelessly transmitted to the retinal prosthesis. This offers life-changing abilities to those currently unable to see anything except, at best, extremely bright lights. Although the resulting vision is not the same as when these patients had normal vision, investigators involved in the clinical trials are eager about the approval. Professor Mark Humayun, University of Southern California: "It is incredibly exciting to have FDA approval to begin implanting the Argus II and provide some restoration of vision to patients blinded from RP. In the patients that have been implanted to date, the improvement in the quality of life has been invaluable. The fact that many patients can use the implant in their activities of daily living such as recognising large letters, locating the position of objects, and more, has been beyond our wildest dreams, yet the promise to the patients is real and we expect it only to improve over time." This announcement follows receipt of the European approval in 2011, and a unanimous recommendation by the Ophthalmic Devices Advisory Panel in September 2012 that this revolutionary product be made available to US patients. It is the culmination of more than 20 years' research and development, two clinical trials, over $100m in public investment by the National Eye Institute, the Department of Energy, and the National Science Foundation, plus an additional $100m in private investments.
With approval from the FDA, the Argus II is slated to be available later this year in clinical centres across the country. Second Sight will be actively adding sites to make the therapy more readily available and encourages interested facilities and patients to contact them. Robert Greenberg, PhD, President and CEO of Second Sight: "We are thrilled to be able to offer the only FDA-approved long-term therapy for people suffering from advanced RP. With this approval, we look forward to building a strong surgical network in the United States and recruiting new hospitals that will offer the Argus II retinal implant. This is a game changer in sight-affecting diseases, that represents a huge step forward for the field and for these patients who were without any available treatment options until now." The Argus II has 60 electrodes implanted in the retina. Research is being undertaken on devices with more electrodes and potentially higher resolution. At the Massachusetts Institute of Technology, for example, one team is developing a system that would feature up to 400 electrodes. Bionic Vision Australia, meanwhile, aims to have a 1,000 electrode bionic eye commercially available by 2019. California's Stanford University has the most ambitious project of all, with a different approach based on tiny photovoltaic cells instead of electrodes, allowing 5,000 to be connected at the back of the eye. Perhaps in the 2030s, we will reach a point where bionic eyes begin to exceed the power and resolution of normal vision. If that happens, even healthy individuals may be tempted to "upgrade" their sight.
Comments »
|