29th November 2014 Breakthrough in treating advanced bladder cancer Researchers at Queen Mary, University of London, have announced "a hugely exciting step forward" in treating advanced bladder cancer.
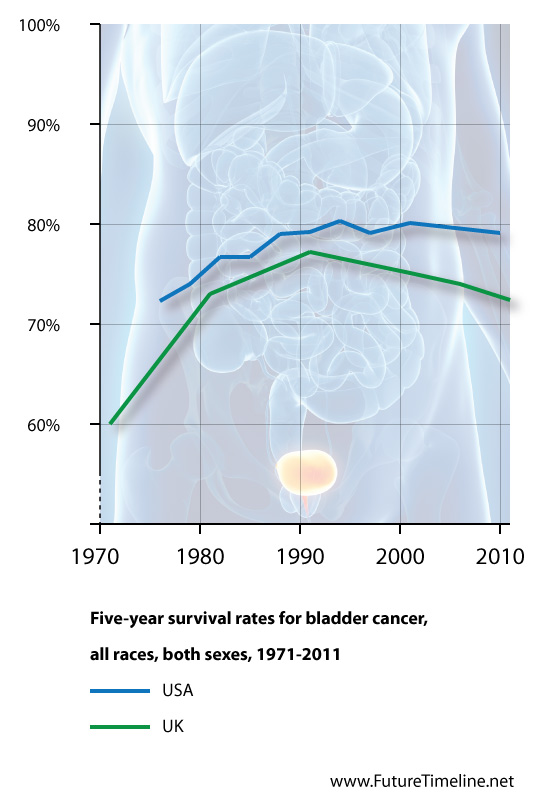
With almost 430,000 new cases each year, bladder cancer is the 9th leading cause of cancer in the world. A significant percentage of diagnoses are advanced (meaning the cancer has already spread to another part of the body), making it very difficult to treat, with chemotherapy the only option. Patients often choose to forgo chemotherapy due to its toxicity and limited survival benefit. As shown in the graph below, no major advances have occurred in treating bladder cancer during the past 30 years. That may be about to change, thanks to research by Queen Mary, University of London. Published this week by the journal Nature, a new study examines an antibody (MPDL3280A) which blocks a protein (PD-L1) thought to help cancer cells evade immune detection. In a Phase one clinical trial, 68 patients with advanced bladder cancer (who had failed all other standard treatments such as chemotherapy) received a cancer immunotherapy medicine – MPDL3280A – which is being developed by Roche. In addition, the patients were all tested for the protein PD-L1 and 30 were identified as having PD-L1 positive tumours. After six weeks of treatment, 43 per cent of PD-L1-positive patients found their tumour had shrunk. This rose to 52 per cent after 12 weeks of follow up. In two of these patients (7 per cent) radiological imaging found no evidence of the cancer at all following the treatment. Patients who had a positive response to treatment found the benefits were prolonged, and safety results were also encouraging, with few reported side effects. The early results of this drug are so promising that it has received a breakthrough therapy designation status by the U.S. FDA. Dr Tom Powles, lead author and Consultant Medical Oncologist at Queen Mary University of London, says: "This study is a hugely exciting step forward in the search for alternative advanced bladder cancer treatment. For decades, chemotherapy has been the only option, with a poor outcome and many patients too ill to cope with it. Not only has this investigational drug had a striking response rate, we can target this therapy for patients by screening specific protein PD-L1. "We now need larger trials to confirm our findings, and as this drug has been given breakthrough designation status by the FDA, we hope to fast track this process so we can begin to give hope to the thousands of people affected by advanced bladder cancer each year."
Comments »
|