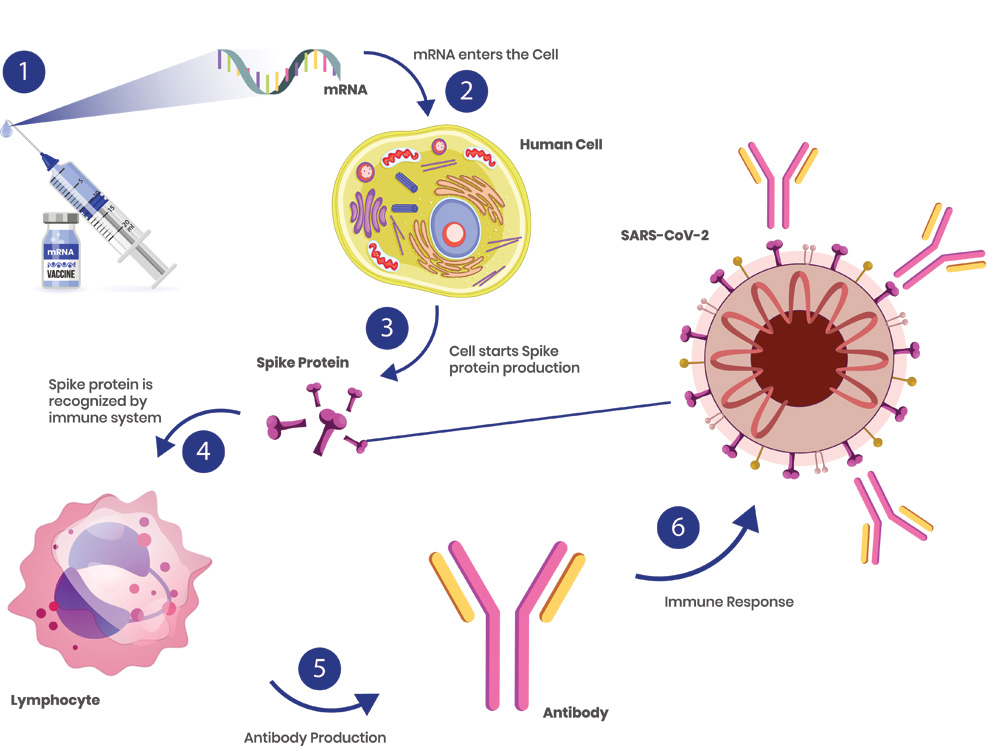
13th September 2021 mRNA cancer therapy enters human trials after success in mice BioNTech, the company that partnered with Pfizer to develop a COVID-19 vaccine, is now testing whether mRNA can be used to treat cancer. Messenger RNA (mRNA) vaccines work by tricking our bodies into producing a small part of a virus. For the Pfizer–BioNTech COVID-19 vaccine, this involved isolating the spike protein of SARS-CoV-2 (the virus that causes COVID-19). This protrudes from the outer surface of the virus and is used to latch onto specific cells in your body, infecting them and causing more copies of the virus to be made. Researchers obtained the spike protein's RNA, then created mRNA based on these molecular instructions. Once injected, the mRNA vaccine will instruct cells to build spike proteins in large volumes – not the virus itself, just the spike protein. This is enough to kickstart our immune response, training the body to recognise the spike protein, without making us sick. B-cells (also known as lymphocytes) can last for months or years and will "remember" the spike protein, making Y-shaped proteins called antibodies to destroy any SARS-CoV-2 encountered in the future.
Credit: Deborah Asamoah
The Pfizer–BioNTech COVID-19 vaccine has been highly effective. A study in The New England Journal of Medicine, published in July, found that two doses have 93.7% effectiveness against symptomatic disease caused by the alpha (B.1.1.7) variant and 88% effectiveness against symptomatic disease caused by the delta (B.1.617.2) variant of coronavirus. Public Health England recently concluded there is 96% effectiveness against hospitalisation. Following this remarkable success, BioNTech has been researching the potential for mRNA in treating other illnesses. The company has just published data in the peer-reviewed journal Science Translational Medicine from a new therapy it is developing for cancer. A team led by BioNTech designed a cocktail of mRNAs that instruct cells to make cytokines. These are small proteins released by cells that are essential in controlling the growth and activity of immune system cells and blood cells. The four types coded by the mRNAs in this study have cancer-fighting abilities and are interleukin-12 (IL-12), interferon-alpha, granulocyte-macrophage colony-stimulating factor, and IL-15 sushi. Previous studies have shown that using cytokines directly in tumours via gene therapy can lead to lingering side effects, due to off-target toxicity and difficulty in achieving local delivery. By contrast, the study authors say, "mRNA is an ideal therapeutic to ensure transient and local translation of cytokines."
The team injected the mRNA into colon and melanoma tumours in 20 mice. The treatment halted tumour growth and caused a complete regression of cancer in 17 (85%) of the animals. They combined that mRNA cocktail with anti-CTLA-4 or anti-PD-1 checkpoint inhibitors, which strengthened the anti-tumour effects and the tumour regression. Checkpoint inhibitors are a relatively new addition to cancer immunotherapies and work by blocking the receptors that cancer cells use to send "off" signals to T-cells. When kept in their "on" state, T cells kill the cancer cells. "Anti-tumour activity extended beyond the treated lesions and inhibited growth of distant tumours and disseminated tumours," the authors add. Although demonstrated in a relatively small (20) cohort of animal models, this combination of cytokines and checkpoint inhibitors appears so effective that it can now proceed to human clinical studies. For the initial study in mice, BioNTech partnered with French pharmaceutical giant, Sanofi. The two companies have now launched a phase 1 trial of the drug, called SAR441000, in patients with solid tumours. This will be tested as both a monotherapy and in combination with Libtayo (a checkpoint inhibitor), involving 231 participants and concluding in 2024.
Comments »
If you enjoyed this article, please consider sharing it:
|