14th August 2018 Key aspect of aging reversed in human cells Scientists in the UK have developed new compounds able to reduce so-called "senescent" cells by up to 50%.
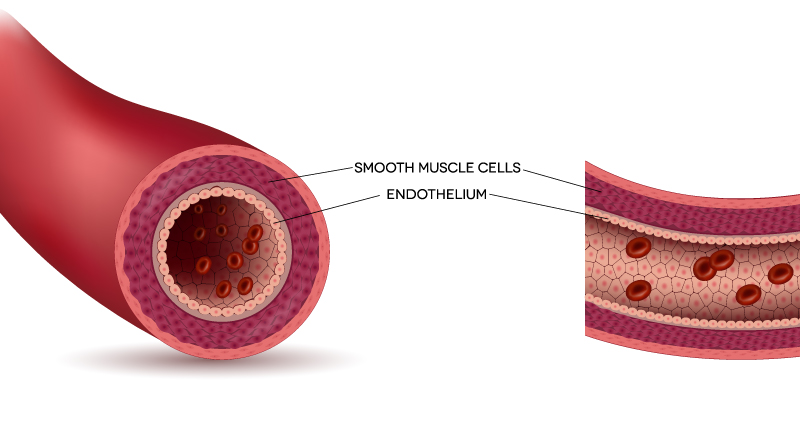
Every day, between 50 and 70 billion cells in your body (or about 0.16% of the total number) allow themselves to die. This form of cell suicide helps to maintain a healthy equilibrium, ensuring an appropriate number of cells are present. Over time, however, this process becomes disrupted by so-called "senescent" cells. These are no longer capable of dividing – but remain in the body as a kind of "zombie" cell. The gradual accumulation of senescent cells results in side effects, including the production of harmful chemicals and interference with nearby healthy cells. This damages the body and is a prime cause of aging. The situation can worsen dramatically if a cell's DNA has been seriously damaged, potentially leading to out-of-control growth and cancer or neurodegenerative disorders. There are many reasons why our tissues and organs stop working, but in recent years, the role of senescent cells has become a major focus for biologists and aging researchers. Removing these old, zombie-like cells has been shown to improve key features of aging in animals. In 2015, for example, a team from the Scripps Research Institute and Mayo Clinic described a new class of drugs, called "senolytics", able to selectively kill senescent cells and potentially restore the balance of cell numbers. Two compounds, given to already old mice, were shown to extend the animals' healthspan, improving their cardiovascular function and exercise endurance, as well as reducing osteoporosis and frailty. Two years later, in 2017, a team of Dutch scientists announced a new drug treatment able to "flush out" senescent cells, again in old mice. This boosted the animals' stamina, restored hair growth and even helped to improve organ function. Also in 2017, researchers at the University of Exeter found a way to actually rejuvenate senescent cells, giving them longer telomeres and the ability to start dividing again. Now, that same team at Exeter has published another study. For their latest work, endothelial cells – which line the inside of blood vessels – were tested with compounds designed to target mitochondria (the "power stations" of cells). The number of senescent cells they contained was reduced by up to 50%. The team also identified two splicing factors (a component of cells) that play key roles in when and how endothelial cells become senescent. These findings raise the possibility of future treatments not only for blood vessels – which become stiffer as they age, raising the risk of problems including heart attacks and strokes – but for other cells too.
"As human bodies age, they accumulate old cells that do not function as well as younger cells," explains Professor Lorna Harries, from the University of Exeter Medical School. "This is not just an effect of aging – it's a reason why we age. The compounds developed at Exeter have the potential to tweak the mechanisms by which this aging of cells happens. We used to think age-related diseases like cancer, dementia and diabetes each had a unique cause, but they actually track back to one or two common mechanisms. This research focuses on one of these mechanisms, and the findings with our compounds have potentially opened up the way for new therapeutic approaches in the future. This may well be the basis for a new generation of anti-degenerative drugs." The compounds in question – AP39, AP123 and RT01 – were designed to deliver tiny quantities of hydrogen sulfide to mitochondria inside the cells. This gas is normally toxic. However, in such minute amounts, using a "molecular postcode" to go directly where needed, it helped the old or damaged cells to generate the energy for survival and to reduce senescence. "Nearly half of the aged cells we tested showed signs of rejuvenating into young cell models," said Professor Harries. "Our compounds provide mitochondria in cells with an alternative fuel to help them function properly," said co-author Professor Matt Whiteman, also from the University of Exeter. "Many disease states can essentially be viewed as accelerated aging, and keeping mitochondria healthy helps either prevent or – in many cases using animal models – reverse this." The team's work appears in the journal Aging.
Comments »
If you enjoyed this article, please consider sharing it:
|