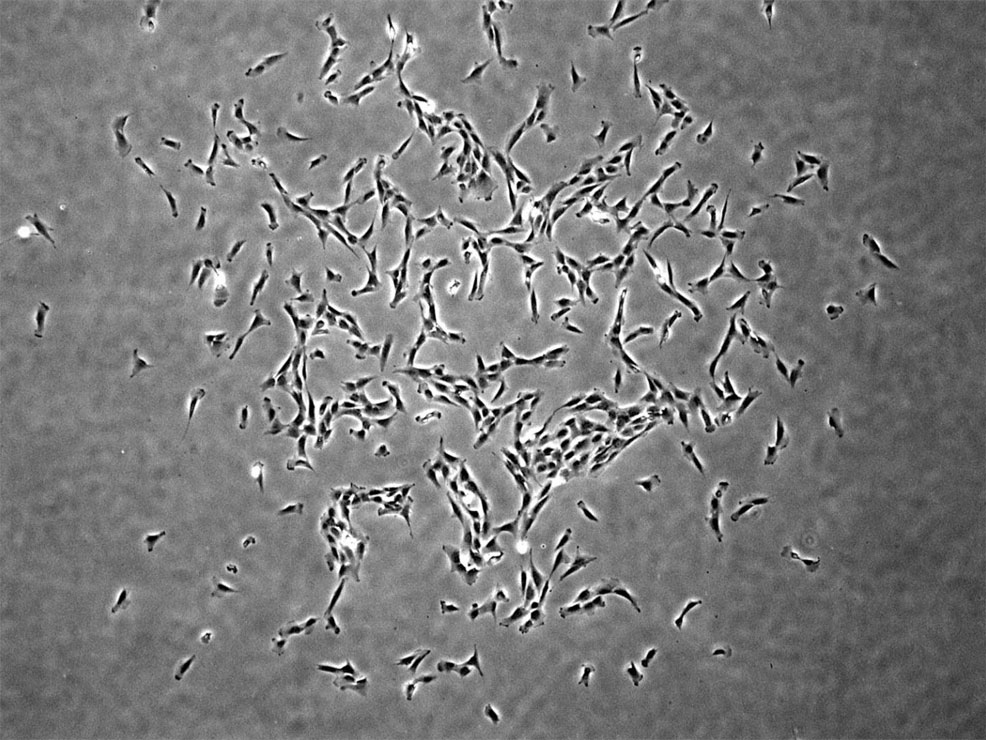
22nd September 2018 Skeletal stem cells identified in humans For the first time, human skeletal stem cells that become bone, cartilage, or stroma cells have been isolated from foetal and adult bones.
Skeletal stem cells have previously been observed in rodent models, but never identified in humans, until now. The researchers were also able to derive them from human induced pluripotent stem cells, opening up new therapeutic possibilities. Their breakthrough is published this week in the journal Cell. "Given the tremendous medical burden imposed by degenerative, neoplastic, post-traumatic, and post-surgical skeletal disorders, we believe that identifying this human skeletal stem cell and elucidating its lineage map will enable the molecular diagnosis and treatment of skeletal diseases," says the senior study author, Michael Longaker, from Stanford University School of Medicine. In the animal kingdom, many vertebrates have skeletal tissues with exceptional regenerative potential. Bone defects heal readily, and some vertebrates can regenerate entire portions of their limbs. But in other vertebrates, these regenerative capacities are more restricted. For example, bones in mice and humans can recover from small- to moderate-sized defects, but adult cartilage tissues possess little to no regenerative ability. In addition, both mice and humans display severe age-related degeneration of skeletal tissues over time. Skeletal dysfunction can lead to a broad spectrum of health conditions – from age-related diseases, such as osteoporosis and osteoarthritis, to non-healing skeletal injury, blood disorders, and even cancer. Despite its significant impact on health and disease, treatment options aimed at improving skeletal function are currently limited. One major hurdle is that stem cell regulation in the human skeletal system remains largely unexplored.
In their new study, Longaker and his collaborators addressed this gap in knowledge, by identifying and characterising human skeletal stem cells and downstream bone and cartilage progeny in a variety of tissues. These self-renewing and multipotent cells were present in both foetal and adult human bone marrow tissues and could be derived from induced pluripotent stem cells (iPSCs). By defining the relationships between human skeletal stem cells and downstream skeletal progenitors, the researchers created a detailed lineage map of stem-cell-mediated formation of skeletal tissues in humans. Moreover, transcriptomic and epigenetic comparisons with mouse skeletal stem cells revealed evolutionarily conserved pathways regulating the stem-cell-mediated formation of skeletal tissues, as well as divergent molecular pathways that may regulate species-specific differences in bone development and skeletal structure. "By comparing the molecular and functional differences in specific types of stem cells between different species of vertebrates, it may be possible to uncover convergent and divergent mechanisms that underlie tissue growth and regeneration – and apply this understanding towards enhancing health and rejuvenation in humans," says co-author Charles Chan, also from Stanford. "This is a major step forward," says John Adams, a molecular biologist and physician at the University of California, Los Angeles. "Whether they can isolate them in large enough quantities to be clinically useful, that's going to take a while to find out."
Comments »
If you enjoyed this article, please consider sharing it:
|