30th September 2018 First human oesophageal tissue grown entirely from pluripotent stem cells In yet another stem cell breakthrough, researchers at Cincinnati Children's Hospital Medical Center report the first human oesophageal tissue grown entirely from pluripotent stem cells.
The oesophagus is a muscular tube that actively passes food from the mouth to the stomach. The organ can be affected by congenital diseases, such as oesophageal atresia, a narrowing or malformation of the oesophagus caused by genetic mutations. Additionally, there are several diseases that can afflict people later in life. Some include oesophageal cancer, gastro-oesophageal reflux disease, or a rare ailment called achalasia that affects the muscles of the lower oesophagus and prevents the passage of food. In the U.S., five-year survival rates for oesophageal cancer are very low – currently around 23%. This figure is unlikely to reach 100% until the year 2100 or later, based on current trends. Improving the treatment of all of these conditions will require a more precise understanding of the genetic and biochemical mechanisms behind their cause. Scientists believe this could be achieved by having the ability to generate and study robust, functional, genetically-matched models of human oesophageal tissue grown from a person's own cells. Now, in a study published by the journal Cell Stem Cell, researchers from Cincinnati Children's Hospital Medical Center (CCHMC) have described using pluripotent stem cells (PSCs) to grow human oesophageal "organoids" – miniature versions of the full-sized organ.
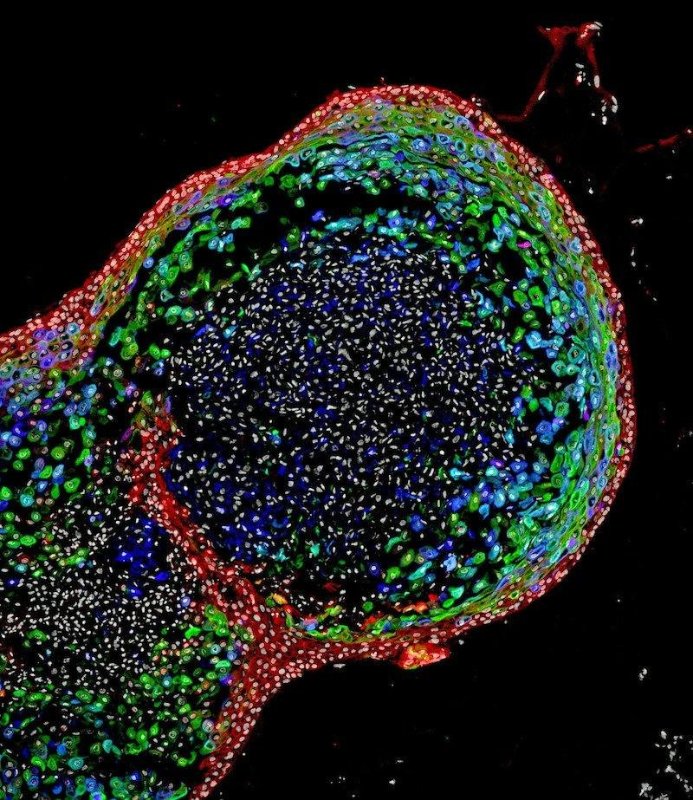
"Disorders of the oesophagus and trachea are prevalent enough in people that organoid models of the human oesophagus could be greatly beneficial," says Jim Wells, PhD, Chief Scientific Officer. "In addition to being a new model to study birth defects like oesophageal atresia, the organoids can be used to study diseases like eosinophilic oesophagitis and Barrett's metaplasia – or to bioengineer genetically-matched oesophageal tissue for individual patients." The scientists based their new method on precisely timed, step-by-step manipulations of genetic and biochemical signals that pattern and form embryonic endoderm and foregut tissues. They focused in part on a gene called Sox2 and its associated protein, which are already known to trigger oesophageal conditions when their function is disrupted. In one test – which confirmed the importance of Sox2 expression on oesophageal formation – researchers studied the complete loss of Sox2 during the embryonic development process in mice. The absence of Sox2 resulted in oesophageal agenesis, a condition in which the oesophagus terminates in a pouch and does not connect to the stomach. Fully formed, human oesophageal organoids were grown in about two months, to lengths of 300-800 micrometres (0.3-0.8 mm) – just large enough to be visible to the naked eye. After successfully generating these, the bioengineered tissues were then compared to oesophageal tissues from patient biopsies. Tests showed that the bioengineered and biopsies tissues were strikingly similar in composition, according to the authors. The research team is now continuing its studies into the bioengineering process for oesophageal organoids and identifying future projects to advance the technology's eventual therapeutic potential, according to Wells. This includes using the organoids to examine the progression of specific diseases and congenital defects affecting the oesophagus.
Comments »
If you enjoyed this article, please consider sharing it:
|