7th May 2023 New drug slows Alzheimer's by 35% Drug company Eli Lilly reports that donanemab can slow the pace of Alzheimer's disease by 35%, following a Phase 3 study in human patients.
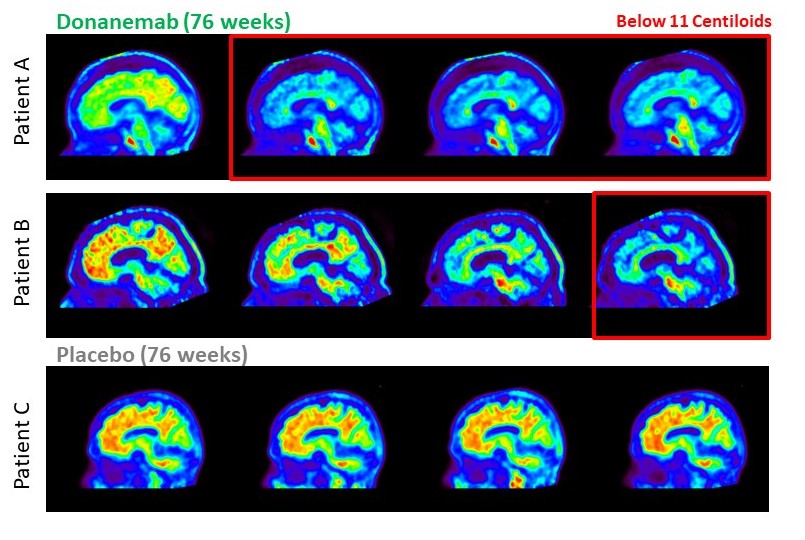
In recent years, Alzheimer's has become an increasingly major public health issue. The prevalence of this disease – a progressive neurological disorder that affects memory, thinking, and behaviour – is being driven by aging populations, changes in lifestyle factors, and improvements in diagnostic techniques. According to a recent report from Alzheimer's Disease International, an estimated 55 million people are now living with dementia, with Alzheimer's disease accounting for up to 70% of cases. The report also predicts that the number of people living with dementia will nearly triple by 2050. This growth in case numbers is predicted to have a significant impact on healthcare systems and economies worldwide, highlighting the urgent need for effective treatments and interventions to prevent or delay the onset of symptoms. In 2013, drug company Eli Lilly began a Phase 1 clinical trial of donanemab, a new monoclonal antibody. This followed earlier tests on mice that had shown promising results. The first human studies achieved similar success, leading to Phase II and III trials. After a decade of research and development, Eli Lilly has now completed the Phase III study and published its results. This latest trial enrolled a much larger group than earlier ones, with 1,736 participants aged 60-85 who had early symptomatic Alzheimer's disease. Donanemab targets a specific form of beta-amyloid protein called N3pG. This form of beta-amyloid is thought to play a key role in the development and progression of Alzheimer's by forming toxic protein aggregates in the brain, leading to neuronal damage and cognitive decline. The exact mechanism by which donanemab promotes clearance of N3pG is not fully understood yet, but is believed to involve the activation of microglia. These are immune cells in the brain that can engulf and clear beta-amyloid deposits. By activating microglia, donanemab may enhance the brain's ability to clear toxic beta-amyloids and improve cognitive function in patients. The Phase III study of donanemab found that people receiving the drug experienced a 35% slower decline, as measured on cognitive tests, than those who only received a placebo. Those taking the drug also showed 40% less decline in their ability to perform daily activities such as driving, managing their finances and holding conversations. A year after beginning treatment, nearly half (47%) of people on donanemab (compared to 29% on placebo) had no clinical progression. "These Phase 3 data confirm the benefit observed in our TRAILBLAZER-ALZ study and show that donanemab, if approved, may represent a significant step forward for people with early symptomatic Alzheimer's, and allow them to continue to participate in activities that are meaningful to them," said Anne White, executive vice president of Eli Lilly and Company and president of Lilly Neuroscience. "We believe our data meets the 'high level of evidence' the Centers for Medicare & Medicaid Services (CMS) has described as the trigger for reconsideration of its National Coverage Determination. People with early Alzheimer's disease need and deserve full coverage and access for approved therapies." With Alzheimer's being notorious for lacking treatment options, donanemab is a significant breakthrough and milestone on the road to a cure. This also follows progress made in 2022, when a separate drug called lecanemab produced a 27% slowdown in cognitive decline, following its Phase III trial. If more studies of new Alzheimer's therapies are published in the coming years – hopefully with ongoing improvement in those percentages – it might be possible to extrapolate a trend to predict when that highly anticipated cure is likely. It will be interesting to see how the recent explosion of AI can influence the pace of research and whether it means a cure is arriving sooner than expected.
Comments »
If you enjoyed this article, please consider sharing it:
|