1st April 2024 Key aspect of aging reversed in mice In a study published by Stanford University, old mice developed more youthful immune systems after treatment with an antibody targeting abnormal stem cells.
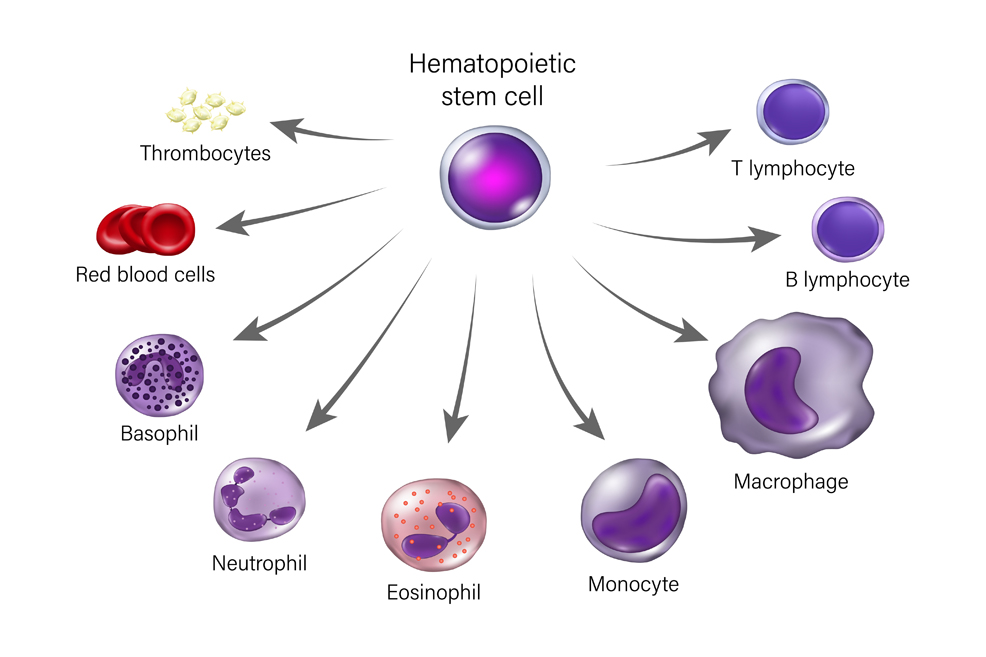
As we age, so too does our immune system. This decline, known as immunosenescence, makes us more susceptible to infections, chronic inflammation, and diseases like cancer. A groundbreaking study from Stanford University, however, has illuminated a promising path forward, showing it is possible to reverse aspects of immune aging through targeted therapies. A new treatment given to mice aged between 18 and 24 months, equivalent to humans aged 56–70, enabled them to fight infections and resist inflammation in a way that resembled younger mice. The results are described in the journal Nature. The study involved hematopoietic stem cells (HSCs), also called blood stem cells, which are thought to play a major role in the progress of immunosenescence. HSCs are responsible for the continuous regeneration of blood cells, including leukocytes (white blood cells) such as the T and B lymphocyte subtypes.
As illustrated above, HSCs can differentiate into various specialised immune cells, serving a critical function in maintaining the immune system throughout the lifespan of an organism. Over time, however, the HSCs diminish in their self-renewal capacity, due to the accumulation of oxidative damage to DNA by aging and cellular metabolic activity, as well as telomere shortening. This causes an imbalance – or "bias" – in the production of some types of immune cells, making the body less able to fight infection and more prone to chronic inflammation, which in turn leads to accelerated aging and a higher risk of heart disease and cancer. A team at Stanford developed a treatment using antibodies to recognise and attack certain blood cells that might be causing this imbalance, while leaving healthier cells untouched. They targeted myeloid cells, which the HSCs begin to favour as we age. This bias is known as "myeloid skew" and results in more myeloid cells (such as neutrophils and monocytes), but fewer lymphoid cells (such as B and T lymphocytes, which are crucial for adaptive immunity). "Older people just don't make many new B and T cell lymphocytes," said Irving Weissman, Professor of Pathology and Developmental Biology at Stanford and Director of the Institute of Stem Cell Biology and Regenerative Medicine. "During the start of the COVID-19 pandemic it quickly became clear that older people were dying in larger numbers than younger people. This trend continued even after vaccinations became available. If we can revitalise the aging human immune system like we did in mice, it could be lifesaving when the next global pathogen arises." Weissman is a pioneer of research on HSCs, having been the first to isolate them in mice and humans in the late 1980s. In the years since, he and his colleagues have investigated the molecular behaviour of these stem cells in ever more detail, painstakingly tracing the complicated relationships among the many cell types they are responsible for.
38% fewer abnormal stem cells
In this latest study, six elderly mice received an injection of the antibody. One week later, they had a 38% lower count of "biased" stem cells, compared to six untreated rodents of the same age. In other words, fewer abnormal stem cells and a greater mix of youthful immune cells. The treated mice had a significantly greater quantity of two types of white blood cells crucial for recognising and combatting pathogens, as well as lower levels of inflammation. "You can think of it as kind of turning back the clock," explained Jason Ross, PhD, study co-author. "We're making the proportion of these [immune] cells more similar to [those of] a younger adult mouse. Not only did we see a shift toward cells involved in adaptive immunity, but we also observed a dampening in the levels of inflammatory proteins in the treated animals. We were surprised that a single course of treatment had such a long-lasting effect. The difference between the treated and untreated animals remained dramatic even two months later." When the treated animals were vaccinated eight weeks later against a virus they hadn't encountered before, their immune systems responded more vigorously than those of untreated animals, and they were significantly more able to resist infection by that virus. "Every feature of an aging immune system – functional markers on the cells, the prevalence of inflammatory proteins, the response to vaccination and the ability to resist a lethal infection – was impacted by this single course of treatment targeting just one cell type," added Ross. According to Weissman, it will be at least three to five years before his team can begin testing the antibody in human patients. Replicating the success of the animal models is by no means guaranteed. But there are similarities between mice and humans in terms of stem cell biology and the production of immune cells. "It's a really important first step," said Robert Signer, a stem cell biologist at the University of California, San Diego, who was not involved in the research. "I'm excited to see where they take this work next." Aubrey de Grey, who coined the term longevity escape velocity in 2004, described the study as "very exciting" and noted that Weissman's team has achieved the "first practical way to reverse myeloid skew, a key aspect of immune system aging."
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||