14th March 2025 Scientists solve decades-long Parkinson's mystery Australian researchers have made a leap forward in the fight against Parkinson's disease, solving a decades-long mystery that paves the way for development of new drugs to treat the condition.
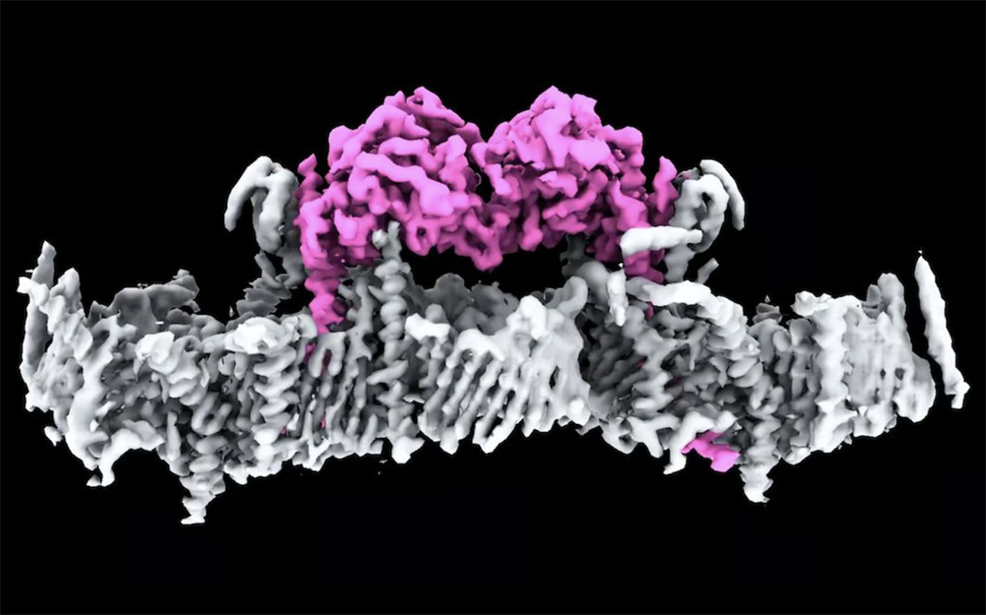
First discovered more than 20 years ago, PINK1 is a protein directly linked to Parkinson's disease – the fastest growing neurodegenerative condition in the world. Until now, no one had seen what human PINK1 looks like, how PINK1 attaches to the surface of damaged mitochondria, or how it is switched on. In a major breakthrough, researchers at the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne, Australia, have determined the first ever structure of human PINK1 bound to mitochondria. This work, published yesterday in Science, could help find new treatments for the condition that currently has no cure or drug to stop its progression. Parkinson's is the second most common neurodegenerative disease after Alzheimer's. The condition is insidious, often taking years, and sometimes even decades to diagnose. Often associated with tremors, there are close to 40 symptoms including cognitive impairment, speech issues, body temperature regulation and vision problems. In Australia, over 200,000 people live with Parkinson's and between 10% and 20% have Young Onset Parkinson's Disease – meaning they are diagnosed under the age of fifty. The impact of Parkinson's on the Australian economy and healthcare systems is estimated to be over $10 billion each year. Mitochondria are cellular components, often described as the "powerhouses of the cell", and produce energy in all living things. Cells that require a lot of energy can contain hundreds or thousands of mitochondria. The PARK6 gene encodes the PINK1 protein, which supports cell survival by detecting damaged mitochondria and "tagging" them for removal. Below is a world-first image of two PINK1 proteins attached to the membrane of a mitochondrion.
In a healthy person, when mitochondria are damaged, PINK1 gathers on mitochondrial membranes and signals through a small protein called ubiquitin that the broken mitochondria need to be removed. This process, known as mitophagy, prevents the buildup of toxins inside cells. However, in individuals with Parkinson's who have a PINK1 mutation, mitophagy fails to function correctly. As a result, toxins accumulate, eventually killing the cell. Brain cells, which require significant energy and are particularly sensitive, become especially vulnerable. This cell death is a hallmark of Parkinson's disease. Although PINK1 has been linked to Parkinson's, and in particular Young Onset Parkinson's Disease, researchers had been unable to visualise it and did not understand how it attaches to mitochondria and is switched on. Professor David Komander, corresponding author on the study, said years of work by his team have now finally unlocked the mystery of what human PINK1 actually looks like, and how it assembles on mitochondria to be switched on. "This is a significant milestone for research into Parkinson's. It is incredible to finally see PINK1 and understand how it binds to mitochondria," said Komander. "Our structure reveals many new ways to change PINK1, essentially switching it on, which will be life-changing for people with Parkinson's." Dr Sylvie Callegari, lead author, explained that PINK1 works in four distinct steps. The first two steps had never been seen before. First, the PINK1 senses mitochondrial damage. Then it attaches to damaged mitochondria. Once attached, it tags ubiquitin, which then links to a protein called Parkin so that the damaged mitochondria can be recycled. "This is the first time we've seen human PINK1 docked to the surface of damaged mitochondria, and it has uncovered a remarkable array of proteins that act as the docking site," said Dr Callegari. "We also saw, for the first time, how mutations present in people with Parkinson's disease affect human PINK1." The idea of using PINK1 as a target for potential drug therapies has long been touted but not yet achieved, because the protein's structure and how it attaches to damaged mitochondria were unknown. The researchers at WEHI used cryo-electron microscopes to obtain the image above. These devices have been around since the 1980s, but recent advances – especially in electron detector technology and image processing algorithms – have dramatically enhanced their resolution. Modern cryo-electron microscopes can capture structures at near-atomic detail, making it possible to visualise proteins like PINK1 in their natural state with much greater clarity than before. The team at WEHI now hope to use this knowledge to find a drug to slow or stop Parkinson's in patients with a PINK1 mutation.
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||