11th August 2013 Deep brain stimulation device is the first to sense and record neural activity while delivering therapy A new automated medical system has initiated research that could one day radically improve how neurological and psychological diseases are treated.
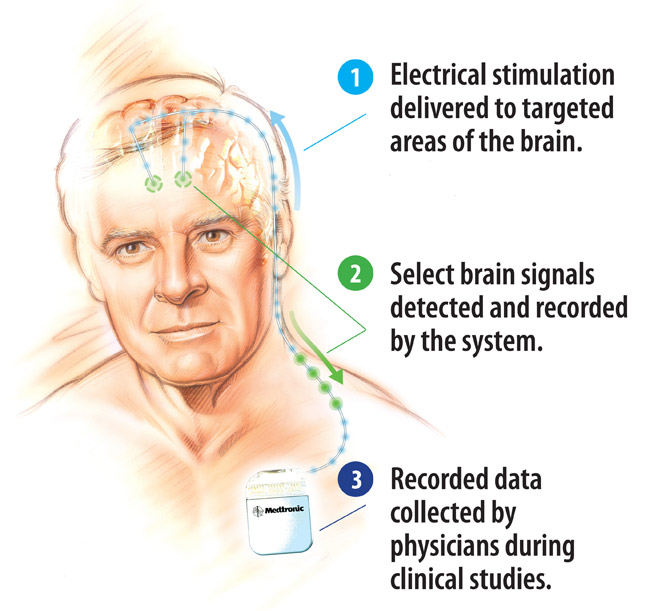
Medtronic, Inc. has announced the first implant of a novel deep brain stimulation (DBS) system that – for the first time – enables the sensing and recording of select brain activity while simultaneously providing targeted DBS therapy. This will initiate research on how the brain responds to the therapy and could yield major insights that significantly change the way people with devastating neurological and psychological disorders are treated. The Activa PC+S DBS system delivers proven Medtronic DBS therapy, while at the same time sensing and recording electrical activity in key areas of the brain, using sensing technology and an adjustable algorithm, which enable the system to gather brain signals at various moments as selected by a physician. Initially, this new technology will be made available to a select group of physicians worldwide for use in clinical studies. These physicians will use the system to map the brain’s responses to Medtronic DBS therapy and explore applications for the treatment across a range of neurological and psychological conditions. The Activa PC+S system was implanted for the first time at Ludwig Maximilians University in Munich, Germany, in a person with Parkinson’s disease. This patient will be treated by a team that includes the neurologist Kai Bötzel and neurosurgeon Jan Mehrkens. Dr. Bötzel will be the first to use data gathered by the Activa PC+S system to gain unprecedented insight into how the brain responds.
Conventional DBS therapy uses an implanted medical device, similar to a pacemaker, to deliver mild electrical pulses to precisely targeted areas of the brain. This needs to be programmed and adjusted by a trained clinician to maximise symptom control and minimise side effects. "Everything that is on the market today is a one-way stimulator," says Joseph Neimat, a neurosurgeon at Vanderbilt University Medical Center who specialises in deep brain stimulation. "The devices don't record or respond to a patient. What would be better would be to have a system that could anticipate or read a patient's state, and then respond with an appropriate stimulus." “DBS therapy works for people with Parkinson’s disease and other movement disorders, but there is much to learn about how the brain responds to the therapy,” said Dr. Bötzel. “This new system will allow us to treat patients with conventional DBS therapy, while at the same time opening the door for research that was not possible until now. We hope these insights will lead to the development of effective new treatments tailored to the needs of individuals.” “Devastating conditions like Parkinson’s disease and obsessive-compulsive disorder take a significant toll on countless people, as well as their loved ones,” said Lothar Krinke, Ph.D., vice president and general manager. “Medtronic is excited to provide this new system to researchers worldwide, and we expect that their respective studies will lead to accelerated understanding of how neurological and psychological conditions develop and progress. This represents a significant milestone for DBS therapy and the long-term journey toward a closed-loop DBS system, which could personalise therapy by using device data to automatically adjust to the needs of individual patients.” Medtronic’s Activa PC+S system received CE (Conformité Européenne) mark in January 2013. It is not approved by the U.S. Food and Drug Administration for commercial use in the United States, and will be made available to select physicians for investigational use only. Additional implants of the Activa PC+S system, including the first implant in the United States, will take place in the coming months.
Comments »
|