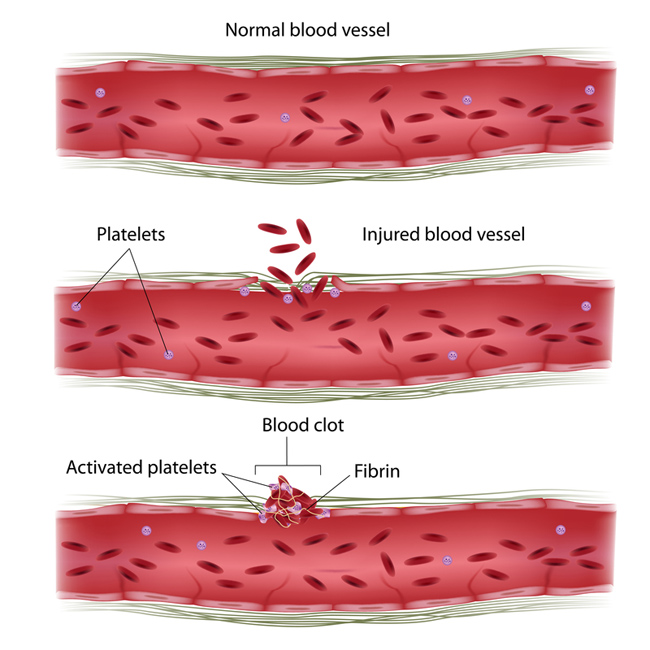
10th November 2014 Synthetic platelets could accelerate healing of injuries Basic wound healing has been advanced with a synthetic platelet that accumulates at sites of injury, clots and stops bleeding three times faster. The synthetic platelets have realistic size, disk-shape, flexibility, and the same surface proteins as real platelets.
Artificial platelets made by the University of California and Case Western Reserve University have been shown to halt bleeding in mouse experiments much faster than nature can on its own. For the first time, they have been able to integratively mimic the shape, size, flexibility and surface chemistry of real blood platelets on albumin-based particle platforms. The researchers believe these four design factors together are vital for inducing clots to form faster at vascular injury sites while preventing harmful clots from forming elsewhere in the body. The new technology, reported in the journal ACS Nano, is aimed at stemming bleeding in patients suffering from traumatic injury, undergoing surgeries or suffering clotting disorders from platelet defects or a lack of platelets. Further, it could be used to deliver drugs to target sites in patients suffering atherosclerosis, thrombosis or other platelet-involved pathologic conditions. Anirban Sen Gupta, associate professor of biomedical engineering at Case Western Reserve, previously designed peptide-based surface chemistries that mimic the clot-relevant activities of real platelets. Building on this work, he now focuses on incorporating morphological and mechanical cues that are naturally present in platelets to further refine their design. "Morphological and mechanical factors influence the margination of natural platelets to the blood vessel wall, and only when they are near the wall can the critical clot-promoting chemical interactions take place," he said. These cues motivated Sen Gupta to team up with Samir Mitragotri, a professor of chemical engineering at the University of California. In his laboratory, Mitragotri has recently developed albumin-based technologies to mimic the geometry and mechanical properties of red blood cells and platelets. Together, the team has developed artificial platelet-like nanoparticles (PLNs) that combine morphological, mechanical and surface chemical properties of natural platelets.
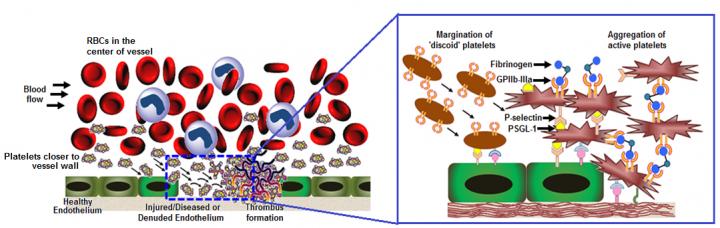
The researchers believe this refined design can simulate natural platelet's ability to collide effectively with larger and softer red blood cells in systemic blood flow. The collisions cause "margination" – pushing the platelets out of the main flow and closer to the blood vessel wall – increasing the probability of them interacting with an injury site. The surface coatings enable the artificial platelets to anchor to injury-site-specific proteins, von Willebrand Factor and collagen, while inducing the natural and artificial platelets to aggregate faster at the injury site. Testing in mouse models showed that injection of the artificial platelets formed clots at the site of injury three times faster than natural platelets alone in the control mice. The ability to interact selectively with injury site proteins, as well as remaining mechanically flexible like natural platelets, enables these artificial versions to safely ride through the smallest of blood vessels without causing damage. Albumin, a protein found in blood serum and eggs, is already used in cancer drugs and considered a safe material. Artificial platelets that don't become involved in a clot and continue to circulate are metabolised within one to two days. The researchers believe their new artificial platelet design may be even more effective in larger volume flows where margination to the blood vessel wall is more prominent. They will soon begin testing that capability. In addition to stemming bleeding, Sen Gupta believes this technology could also be useful in delivering clot-busting medicines directly to clots, to treat heart attack or stroke without having to systemically suspend the body's coagulation mechanism. The artificial platelets may also be used to deliver cancer medicines to metastatic tumours with high platelet interactions.
Comments »
|