22nd April 2016 Ultra-long carbyne stronger than graphene is synthesised for the first time An international team of scientists reports synthesising ultra-long carbyne inside double-walled nanotubes. This exotic form of carbon is even stronger than graphene.
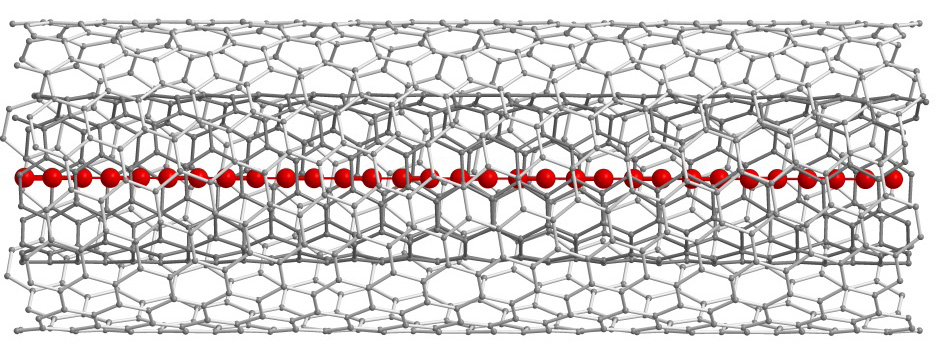
European researchers have successfully stabilised chains of more than 6,400 carbon atoms, using double-walled nanotubes. In a study, published by the journal Nature Materials, they demonstrate a new route for the production of carbyne – infinitely long carbon chains whose mechanical properties surpass those of diamond and graphene. Their method involved using double-walled carbon nanotubes, as shown in these illustrations, to protect the carbon chain from extreme instability in ambient conditions. Elemental carbon appears in many different forms, some of which are very well-known and have been thoroughly studied: diamond, graphite, graphene, fullerenes, nanotubes and carbyne. Within this "carbon family", carbyne (a truly one-dimensional carbon structure) is the only one that has not been synthesised until now, despite having been studied for more than 50 years. Chemists across the world had been trying to synthesise increasingly longer carbyne chains by using stabilising agents, but the longest chain obtained so far (achieved in 2010) was only 44 carbon atoms. Now, a research group at the University of Vienna, led by Prof Thomas Pichler, has presented a new, simple means for stabilising these carbon chains with a record-breaking length of more than 6,400 carbon atoms. The previous record has therefore been broken by two orders of magnitude. To do this, they used the confined space inside a double-walled carbon nanotube as a "nano-reactor" to make the ultra-long carbon chains grow, while providing the chains great stability. This could be tremendously important for future applications.
The researchers unambiguously confirmed the existence of these chains by means of structural and optical probes. This direct experimental proof can be seen as a promising step towards obtaining perfectly linear carbon chains, the researchers' final objective. Theoretical studies have shown that after having linear chains grow inside a carbon nanotube, the hybrid system could have a metallic nature – making it possible to control electronic properties. In other words, this new system is not only interesting from a chemical point of view, it could also be very important in the field of nano devices. According to theoretical models, carbyne has mechanical properties unmatched by any known material. It has twice the tensile stiffness of graphene and nearly three times that of diamond. Furthermore, its electronic properties point towards new nano-electronic applications, such as the development of new magnetic semiconductors, high power density batteries, or in quantum spin transport electronics (spintronics). However, the researchers point out that to achieve this would require extracting these ultra-long, linear carbon chains from the double-walled nanotube containing them and stabilising them in a liquid environment. ---
Comments »
|