Researchers define key events early in the process of cellular aging 22nd November 2012 A new study brings unprecedented clarity to the aging process and provides a paradigm for studying how genes and the environment – including calorie restriction – may influence lifespan.
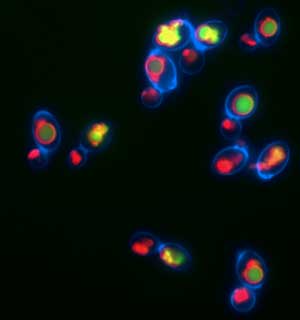
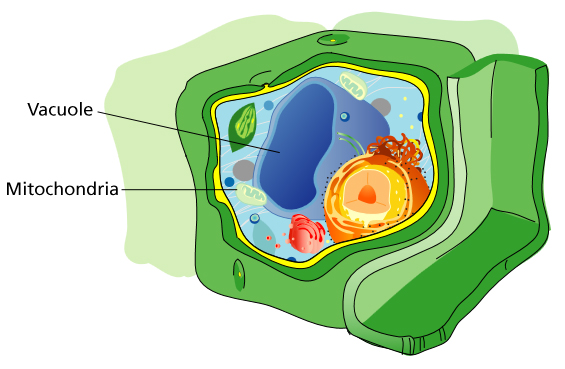
For the first time, scientists at Fred Hutchinson Cancer Research Center – one of the world's leading cancer research institutes – have defined key events that take place early in the process of cellular aging. The discoveries, made through experiments in yeast, bring unprecedented clarity to the complex cascade of events that comprise the aging process. This work paves the way to understanding how genetics and environmental factors interact to influence lifespan, aging and age-related diseases such as cancer and neurodegenerative disorders. The findings – including unexpected results that link aspects of aging and lifespan to a mechanism cells use to store nutrients – were published online yesterday in Nature by co-authors Daniel Gottschling, PhD, a member of the Hutchinson Center's Basic Sciences Division, and Adam Hughes, PhD, a postdoctoral fellow in the Gottschling Lab. Acidity of the vacuole is critical The researchers found the acidity of a structure in yeast cells known as the vacuole is critical to aging and the functioning of mitochondria – the power plants of the cell. They also describe a novel mechanism, which may have parallels in human cells, by which calorie restriction extends lifespan. The work began with Hughes and Gottschling searching for the source of age-related damage in mitochondria. "Normally, mitochondria are beautiful, long tubes, but as cells get older, the mitochondria become fragmented and chunky," said Gottschling. "The changes in shape seen in aging yeast cells are also observed in certain human cells, such as neurons and pancreatic cells, and those changes have been associated with a number of age-related diseases in humans." What causes mitochondria to become distorted and dysfunctional as cells age had long been a mystery, but Gottschling and Hughes discovered that specific changes in the vacuole lead directly to their malfunctioning. The vacuole – and its counterpart in humans and other organisms, the lysosome – has two main jobs: degrading proteins and storing molecular building blocks for the cell. To perform those jobs, the interior of the vacuole must be highly acidic.
Hughes and Gottschling found that the vacuole becomes less acidic relatively early in the yeast cell’s lifespan and, critically, that the drop in acidity hinders the vacuole’s ability to store certain nutrients. This, in turn, disrupts the mitochondria’s energy source, causing them to break down. Conversely, when Hughes prevented the drop in vacuolar acidity, the mitochondria’s function and shape were preserved and the yeast cells lived longer. “Until now, the vacuole’s role in breaking down proteins was thought to be of primary importance. We were surprised to learn it was the storage function, not protein degradation, that appears to cause mitochondrial dysfunction in aging yeast cells,” Hughes said. The unexpected discovery prompted Hughes and Gottschling to investigate the effects of calorie restriction, which is known to extend the lifespan of yeast, worms, flies and mammals, on vacuolar acidity. They found that calorie restriction – that is, limiting the raw material cells need – delays aging at least in part by boosting the acidity of the vacuole. “Now that we have preliminary evidence in yeast of how calorie restriction extends lifespan, our hope is that it can be translated to higher organisms like humans,” Hughes said. Given the similarities in the fundamental biology of yeast and human cells, the researchers’ newly defined link between what cells “eat” and how they age could shed valuable light on the events that lead to age-related disorders in humans. “There has been a lot in the scientific literature and the general media lately about how what you eat affects the aging process, but it has been incredibly confusing. Now we have a new paradigm for understanding how genetics and environment interact to influence lifespan, aging and age-related diseases. That’s what I’m really excited about,” Gottschling said. Lack of energy breaks down mitochondria Gottschling and Hughes speculate that if the vacuole’s declining acidity limits its ability to store certain nutrients and metabolites, they may build up in the cell, flooding the mitochondria. Overwhelmed, the mitochondria use up all their energy – essentially burning out their motors – taking in the surplus. With no power left to import the proteins they need to maintain their elegant shape and execute their regular duties, the mitochondria literally break down. Gottschling and his colleagues are now investigating this hypothesis in detail. They are also exploring what triggers the initial drop in the vacuole’s acidity. The latter research question is of particular interest, because the researchers found that even though vacuolar acidity drops as mother yeast cells age, the acidity in the vacuoles of their newborn daughter cells is normal. This corresponds to previous findings in the field that all daughter yeast cells have the same potential lifespan, regardless of the age of their mothers. The resetting of the daughter’s vacuolar acidity is the earliest event yet observed in cellular rejuvenation, a phenomenon in which age-related defects are seemingly erased in an organism’s offspring. This could help explain how the act of cell division itself contributes to aging.
Comments »
|