2nd March 2013 Nanomedicine breakthrough: protein 'passport' can evade immune system The body's immune system identifies and destroys foreign objects, whether they are bacteria, viruses, flecks of dirt or splinters. Unfortunately, nanoparticles designed to deliver drugs, and implanted devices like pacemakers or artificial joints, are just as foreign and subject to the same hostile response.
Now, however, researchers at the University of Pennsylvania have discovered a way to provide a "passport" for such therapeutic devices, enabling them to get past the body's security system. Among those who conducted the research was graduate student, Pia Rodriguez. "From your body's perspective," she said, "an arrowhead 1,000 years ago and a pacemaker today are treated the same – as a foreign invader. We'd really like things like pacemakers, sutures and drug-delivery vehicles to not cause inflammatory responses from the innate immune system." The innate immune system attacks foreign bodies in a general way. Unlike the "learned" response of our adaptive immune system, which includes the targeted antibodies formed after a vaccination, the innate immune system tries to destroy everything it doesn’t recognise as being part of the body. Among these defences are mobile cells known as macrophages – literally "big eaters" – that find, engulf and destroy invaders. Proteins in blood serum work alongside macrophages, by adhering to objects in the blood stream and drawing the macrophages' attention. If the macrophage determines these proteins are stuck to a foreign invader, they will eat it or signal other macrophages to form a barrier around it:
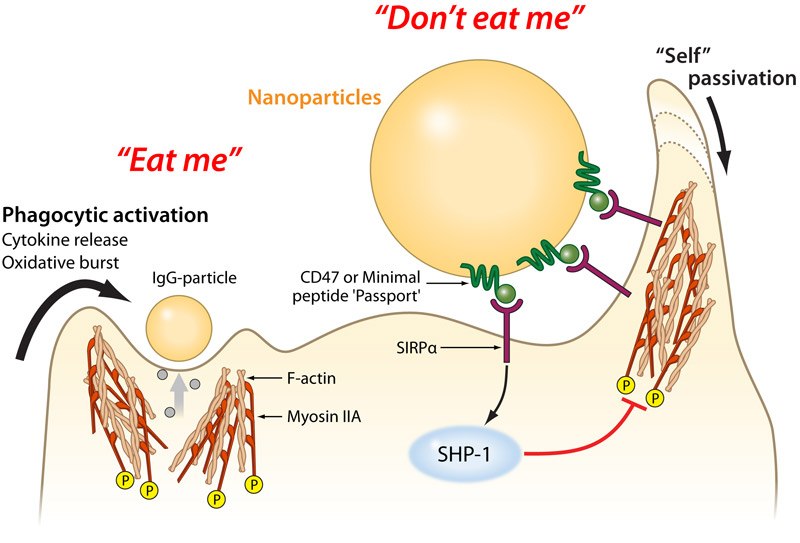
Drug-delivery nanoparticles will naturally trigger this response, so researchers' earlier efforts involved coating them with polymer "brushes." These stick out from the nanoparticle and attempt to physically block various blood serum proteins from attaching to its surface. However, these brushes only slow down the macrophage-signalling proteins. The researchers at Penn University have tried a different approach: convincing the macrophages that the nanoparticles are part of the body and shouldn't be cleared. CD47 – a protein "passport" In 2008, Professor Dennis Discher and colleagues showed that the human protein CD47, found on almost all mammalian cell membranes, binds to a macrophage receptor known as SIRPa. Like a patrolling border guard inspecting a passport, if a macrophage’s SIRPa binds to a cell’s CD47, it tells the macrophage that the cell isn’t an invader and should be allowed to proceed on. Since that study, researchers have determined the combined structure of CD47 and SIRPa together. Using this data, Discher’s group was able to computationally design the smallest sequence of amino acids that would behave like CD47. This “minimal peptide” would have to fold and fit well enough to the receptor of SIRPa to function as a valid passport. After chemically synthesising this minimal peptide, Discher’s team attached it to conventional nanoparticles that could be used in a variety of experiments. Using this method in mice – genetically modified so their macrophages had SIRPa receptors similar to the human version – the researchers demonstrated better imaging of tumours and greater efficacy of anti-cancer drug-delivery nanoparticles. Since the "passport" might be attached to a wide range of drug-delivery vehicles in the future, they also tried antibodies for other types of damaged cells and diseased tissues. Once again, these antibodies served to attract the macrophages' attention and ensure the minimal peptide's passport was being checked and approved.
The success of this method was determined by comparing nanoparticles – with and without a passport – mixed equally and injected into the bloodstream of the mice. Particle ratios, taken after 30 minutes, showed four times as many had survived with a passport than those without. Fluorescent dyes were then used, which allowed tumours to become far more visible and easier to spot, thanks to quadruple the number of nanoparticles reaching their destination. Evading the immune system in this way, and increasing the length of time before nanoparticles are eaten by macrophages will be a major advantage for new treatments. While more research is needed before such applications become a reality, reducing the peptide down to a sequence of only a few amino acids was a critical step. The relative simplicity of synthesising this "passport" molecule makes it very attractive for use in future nanomedicine. "It can be made cleanly in a machine," Discher said, "and easily modified during synthesis in order to attach to all sorts of implanted and injected things, with the goal of fooling the body into accepting these things as 'self.'"
Comments »
|