20th April 2013 Potential new breakthrough in treating ALS Animal trials are set to begin on a gene therapy for ALS – a crippling degenerative condition which counts Stephen Hawking among its sufferers.
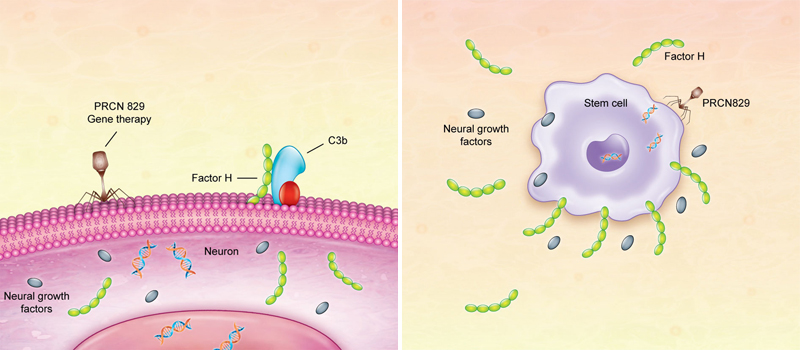
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's, is the most common of the five motor neuron diseases – neurological disorders affecting the cells which control voluntary muscle activity such as walking, speaking, swallowing and general body movement. They are progressive in nature, with increasingly worse disability and, eventually, death. Worldwide, ALS affects 1 to 3 people per 100,000. It usually appears between the ages of 40-60, and is more prevalent in men than women. About 30,000 Americans have the illness, with around 5,600 new cases each year. Half of all patients die within four years of their diagnosis, and only 4% survive longer than 10 years. At present, there is no cure for ALS, and only a single drug – Riluzole – which may increase survival by a few months. Recent efforts have focussed on identifying genes that impact the risk of developing the disease as well as its progression. Neuralgene, a new biotechnology startup company, this week announced that it will begin animal trials in May to study the efficacy of PRCN-829, the first gene therapy for sporadic ALS. This neurotropic, AAV-based platform is built on the earlier stem cell work of Jason Williams, founder and CEO. "Our technology addresses several key aspects of the underlying pathology of ALS," said Leonardo Gonzalez, a researcher for Neuralgene. "In his work, Dr. Williams had identified that production of Factor H by fat-derived mesenchymal stem cells may be a key mode of action."
The new therapy is based on Dr. Williams' discovery that certain proteins produced by stem cells inhibit the attack of ALS. During the development, he added new targets: neural growth factors and a protein implicated in ALS named TDP-43. "When Dr. Williams demonstrated the concept behind stem cells and how to address treatment of ALS using gene therapy, we immediately knew that this was a revolutionary new concept," said Dr. Gonzalez. The PRCN-829 gene therapy is designed to not only target gene delivery to the brain and spinal cord, but also to genetically engineer stem cells. The AAV9 viral vector delivers multiple genes, which include Factor H (a regulator of complement activity), neural growth factors and regulators of TDP-43, to the neural cells. Initial studies have already demonstrated the safety of the gene therapy platform. "The problem with stem cell therapy for ALS is that the results are generally partial and temporary," stated Dr. Williams. "This is because the stem cells produce the growth factors and other proteins for a short period, but then cease. Several stem cell studies have confirmed this. Now with gene therapy, we can increase those factors by a millionfold or greater so that recuperation lasts for many years, or maybe is even lifelong." "ALS is a complex disease with many different underlying causes," continued Dr. Williams. "Our gene therapy will target several of the main underlying mechanisms related to ALS with the hopes of getting a good response in a larger group of patients. However, our platform is versatile, allowing us to change and add different target genes. We expect that soon we will be able to perform a detailed genetic analysis of the patient, identifying their exact underlying cause of ALS. Then we will be able to tailor the therapy to each individual patient." "This is a completely new therapy for ALS, and the groundwork for this technology will lead to the treatment of many other diseases," said Dr. Williams. Neuralgene has several other gene therapies in its research and development pipeline for the treatment of neurodegenerative diseases such as Parkinson's and Multiple Sclerosis (MS). Following initial testing of PRCN-829 in Colombia, Neuralgene plans to seek approval from the FDA for human trials in the United States.
Comments »
|