16th December 2013 Five-fold extension in roundworm lifespan By combining mutations, a five-fold increase in the lifespan of C. elegans has been achieved, the equivalent of a human reaching 400 years of age.
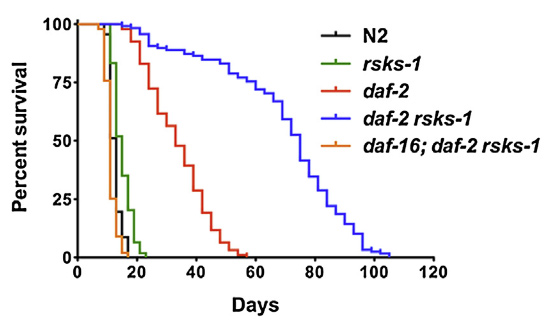
What are the limits to longevity? New research in simple animals suggests that combining mutants can lead to radical lifespan extension. Scientists at Buck Institute combined mutations in two pathways known for lifespan extension and report a synergistic five-fold extension of longevity in the nematode Caenorhabditis elegans. This breakthrough introduces the possibility of combination therapy for aging. The mutations inhibited key molecules involved with insulin signaling (IIS) and the nutrient signaling pathway known as Target of Rapamycin (TOR). Lead scientist, Pankaj Kapahi, PhD, said that mutations in TOR (in this case RSKS-1) usually result in a 30 percent lifespan extension, while mutations in IIS (Daf-2) often result in a doubling of lifespan in the worms – added together, they would be expected to extend longevity by around 130 percent. “Instead, what we have here is a synergistic five-fold increase in lifespan,” he said. “The two mutations set off a positive feedback loop in specific tissues that amplified lifespan. Basically, these worms lived to the human equivalent of 400 to 500 years.”
Kapahi said the research points to the possibility of using combination therapies for aging – similar to what is done for cancer and HIV. “In the early years, cancer researchers focused on mutations in single genes, but then it became apparent that different mutations in a class of genes were driving the disease process,” he said. “The same thing is likely happening in aging.” Kapahi said this research could help explain why scientists are having a difficult time identifying single genes responsible for the long lives experienced by human centenarians. “It’s quite probable that interactions between genes are critical in those fortunate enough to live very long, healthy lives.” Co-author of the study, Di Chen, explained that the positive feedback loop originated in the germline tissue of the worms. The germline is a sequence of reproductive cells that may be passed onto successive generations. “The germline was the key tissue for the synergistic gain in longevity – we think it may be where the interactions between the two mutations are integrated. The finding has implications for similar synergy between the two pathways in more complex organisms.”
Kapahi said the research would ideally move into mice, as a way of determining if the lifespan-extending synergy extends into mammals. “The idea would be to use mice genetically engineered to have suppressed insulin signaling, and then treat them with the drug rapamycin, which is well-known to suppress the TOR pathway.” The team's work is published in Cell Reports.
Comments »
|