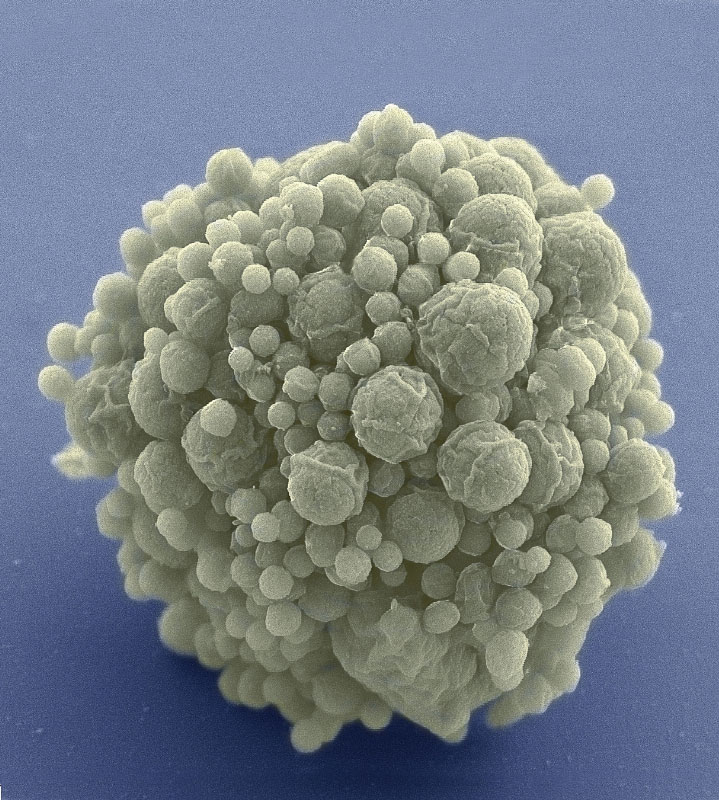
29th March 2016 World's first minimal synthetic bacterial cell Craig Venter's team has synthesised a minimal bacterial genome, containing only the genes necessary for life, and consisting of just 473 genes. This builds upon their earlier research that synthesised Mycoplasma laboratorium in 2010.
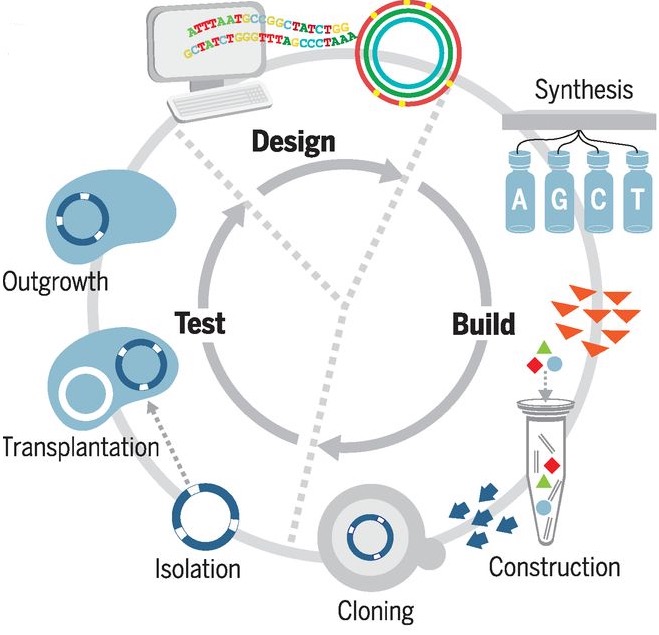
A newly created synthetic organism, pictured above, has been announced by Dr. Craig Venter and his team of researchers, whose earlier work includes the Human Genome Project and Mycoplasma laboratorium. Known as JCVI-syn3.0, it contains just 473 genes, which makes it the world's first minimal synthetic bacterial cell, and the smallest genome of any organism that can be grown in laboratory media. For comparison, the earlier JCVI-syn1.0 (synthesised in 2010) contained 901 genes, while a human cell has over 20,000 genes. Nearly one-third (149) of this organism's genes are of unknown biological function, suggesting the presence of undiscovered functions that are essential for life. World-renowned geneticist Dr. Venter explains: "Our attempt to design and create a new species – while ultimately successful – revealed that 32% of the genes essential for life in this cell are of unknown function, and showed that many are highly conserved in numerous species. All the bioinformatics studies over the past 20 years have underestimated the number of essential genes by focusing only on the known world. This is an important observation that we are carrying forward into the study of the human genome." In 2010, Dr. Venter and his team created the first artificial cell (JCVI-syn1.0), providing proof of principle that genomes can be designed in the computer, chemically made in the lab, and transplanted into a recipient cell to produce a new, self-replicating organism controlled only by the synthetic genome. They then set about their ultimate objective: to synthesise a minimal cell containing only the genes necessary for life in its simplest form, an effort that could help scientists understand the function of every essential gene in a cell. To achieve this, Venter and colleagues again turned to Mycoplasma. They designed minimal genomes in eight different segments, each of which could be tested to accurately classify constituent genes as "essential" or not. During this design-build-test process, quasi-essential genes were also identified – those needed for robust growth, but not absolutely required for life. They whittled away at the synthetic, reduced genome, repeating experiments until no more genes could be disrupted and the genome was as small as possible. Their study also revealed some genes initially classified as "non-essential" do in fact perform the same essential function as a second gene; thus, one of the pair of genes needs to be retained in the minimal genome.
A paper describing the organism is published in the journal Science. The team hopes to decode the unknown 149 genes in the future. They conclude that a major outcome of this minimal cell program is new tools and semi-automated processes for whole genome synthesis. "This paper represents more than five years of work by an amazingly talented group of people," says co-author Dr. Clyde Hutchison. "Our goal is to have a cell for which the precise biological function of every gene is known." "This paper signifies a major step toward our ability to design and build synthetic organisms from the bottom up with predictable outcomes," says Daniel Gibson, PhD, an associate professor at the J. Craig Venter Institute (JCVI). "The tools and knowledge gained from this work will be essential to producing next-generation production platforms for a wide range of disciplines." In the future, synthetic organisms could be used for industrial applications to create revolutionary new medicines, biochemicals, biofuels, agricultural processes and much more.
---
Comments »
|