26th February 2019 Gene therapy cures deafness in mice Scientists have created a new gene therapy to restore hearing in an adult mouse model of DFNB9 deafness.
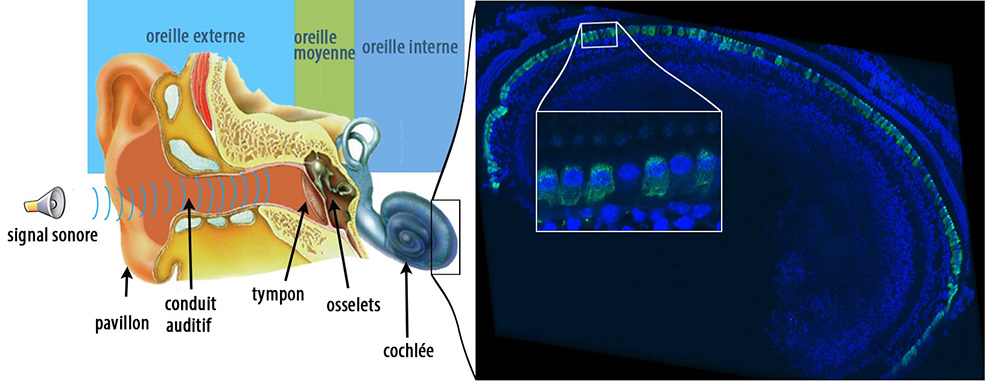
Scientists from the U.S. and France have managed to restore hearing in an adult mouse model of DFNB9 deafness – a hearing disorder that represents one of the most frequent cases of congenital genetic deafness. Individuals with DFNB9 are profoundly deaf, as they are deficient in the gene coding for otoferlin, a protein essential for transmitting sound information at the auditory sensory cell synapses. By carrying out an intracochlear injection of this gene in an adult mouse model, the scientists successfully restored auditory synapse function and hearing thresholds to a near-normal level. These findings, published in the journal PNAS, open up new avenues for future gene therapy trials in human patients with DFNB9. More than half of nonsyndromic profound congenital deafness cases have a genetic cause, and most (80%) of these cases are due to autosomal recessive forms of deafness (DFNB). In humans, DFNB9 represents up to 8% of all cases of congenital genetic deafness. Cochlear implants are currently the only option for recovering hearing in these patients. Adeno-associated viruses (AAVs) are among the most promising vectors for therapeutic gene transfer to treat human diseases. AAV-based gene therapy is a promising option for deafness, but its application is limited by a potentially narrow therapeutic window. In humans, inner ear development is completed in utero and hearing becomes possible at approximately 20 weeks of gestation. In addition, genetic forms of congenital deafness are generally diagnosed during the neonatal period. Gene therapy approaches in animal models must therefore take this into account, and gene therapy efficacy must be demonstrated following a gene injection when the auditory system is already in place. In other words, therapy must reverse existing deafness. In addition, since AAVs have limited DNA packaging capacity (approximately 4.7 kilobase (kb)), it is difficult to use this technique for genes whose coding region (cDNA) exceeds 5 kb – such as the gene coding for otoferlin, which has a 6 kb coding region. To overcome that limitation, the scientists in this study adapted an approach known as dual AAV strategy, so named because it uses two different recombinant vectors, one containing the 5’-end and the other the 3’-end of the otoferlin cDNA. A single intracochlear injection of the vector pair in adult mutant mice was used to reconstruct the otoferlin coding region, leading to long-term restoration of otoferlin expression in the inner hair cells, and then restored hearing. Based on their results, the scientists believe that the therapeutic window for local gene transfer in patients with DFNB9 could be wider than thought, which offers hope of extending this treatment to other forms of deafness.
Comments »
If you enjoyed this article, please consider sharing it:
|