2nd November 2019 New system captures CO2 at any concentration A new carbon capture system has been demonstrated by MIT, which can work on the gas at almost any concentration, using electrodes combined with carbon nanotubes.
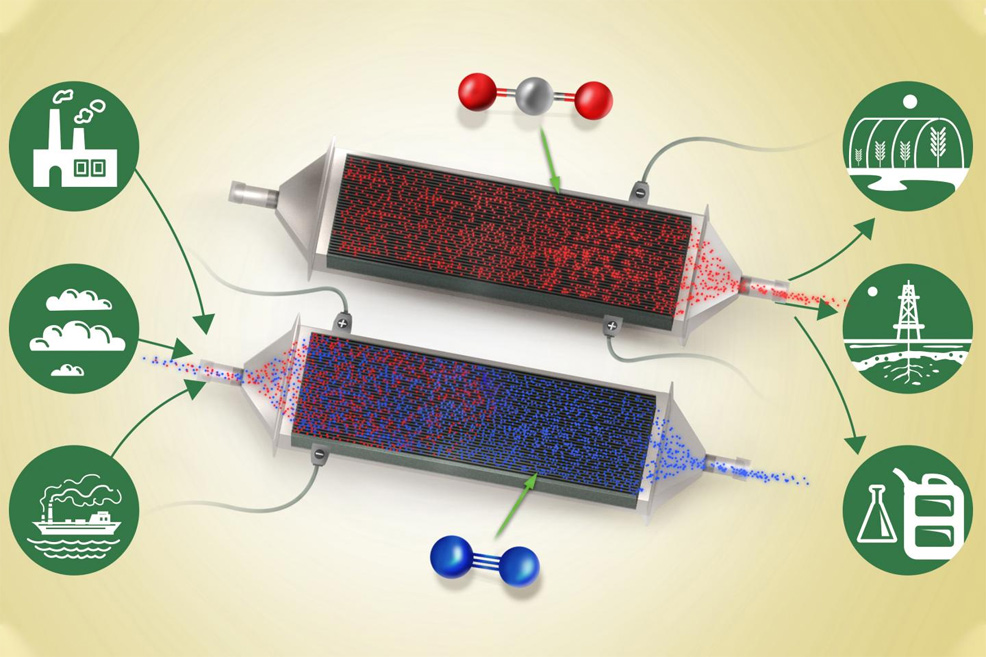
A new way of removing carbon dioxide (CO2) from a stream of air could provide a significant tool in the battle against climate change. The new system can work on the gas at virtually any concentration level, even down to the roughly 400 parts per million currently found in the atmosphere. Most methods of removing carbon dioxide from a stream of gas require higher concentrations, such as those found in the flue emissions from fossil fuel-based power plants. A few variations have been developed that can work with the lower concentrations found in air. However, this latest new method is significantly less energy-intensive and expensive, the researchers say. The technique, based on passing air through a stack of charged electrochemical plates, is described in the journal Energy and Environmental Science by MIT postdoc Sahag Voskian, who developed the work during his PhD, and T. Alan Hatton, Professor of Chemical Engineering. The device is essentially a large, specialised battery that absorbs CO2 from the air (or another gas stream) passing over its electrodes as it is being charged up, and then releases the gas as it is being discharged. In operation, the device would simply alternate between charging and discharging, with fresh air or feed gas being blown through the system during the charging cycle, and then the pure, concentrated CO2 being blown out when discharging – for use in various commercial applications, or storage underground.
As the battery charges, an electrochemical reaction occurs at the surface of the electrodes. These are coated with a compound called polyanthraquinone, which is composited with carbon nanotubes. The electrodes have a natural affinity for carbon dioxide and readily react with its molecules in the airstream or feed gas, even when it is present at very low concentrations. The reverse reaction takes place when the battery is discharged – during which the device can provide part of the power needed for the whole system – and in the process ejects a stream of pure carbon dioxide. The whole system operates at room temperature and normal air pressure. "The greatest advantage of this technology over most other carbon capture or carbon absorbing technologies is the binary nature of the adsorbent's affinity to carbon dioxide," explains Voskian. In other words, the electrode material, by its nature, "has either a high affinity or no affinity whatsoever," depending on the battery's state of charging or discharging. Other reactions used for carbon capture require intermediate chemical processing steps, or the input of significant energy such as heat, or pressure differences. This technology has a number of potential applications, according to Voskian. For example, in soft-drink bottling plants, fossil fuels are often burned to generate the carbon dioxide needed to give the drinks their fizz. Similarly, some farmers burn natural gas to produce carbon dioxide to feed their plants in greenhouses. This new device could eliminate that need for fossil fuels, while actually removing the greenhouse gas right out of the air. Alternatively, the pure carbon dioxide stream could be compressed and injected underground for long-term disposal. This new process of capturing and releasing CO2 "is revolutionary" and "a clear demonstration of the power of electrochemical approaches that require only small swings in voltage to drive the separations," says Voskian. "All of this is at ambient conditions – there's no need for thermal, pressure, or chemical input. It's just these very thin sheets, with both surfaces active, that can be stacked in a box and connected to a source of electricity."
In the lab, the team has proven the system can withstand at least 7,000 charging-discharging cycles, with a 30% loss in efficiency over that time. They are confident of increasing that to 50,000 cycles. The electrodes themselves can be manufactured by standard chemical processing methods. While today this is done in a laboratory setting, it can be adapted so that ultimately they could be made in large quantities through a roll-to-roll manufacturing process similar to a newspaper printing press, Voskian says. "We have developed very cost-effective techniques," he explains, estimating that it could be produced for something like tens of dollars per square metre of electrode. Compared to existing carbon capture technologies, this system has impressive energy efficiency, using only a gigajoule of energy per ton of CO2 captured, consistently. Other current methods have energy consumption varying between 1 and 10 gigajoules per ton, depending on the inlet gas concentration. The researchers have set up a company called Verdox to commercialise the process and hope to develop a pilot-scale plant within a few years. And the system is very easy to scale up, says Voskian: "If you want more capacity, you just need to make more electrodes."
Comments »
If you enjoyed this article, please consider sharing it:
|