3rd March 2021 Nanoparticle cuts cholesterol by 57% Scientists have used lipid nanoparticles to deliver CRISPR genome editing into mouse livers, resulting in a 57% reduction of cholesterol levels.
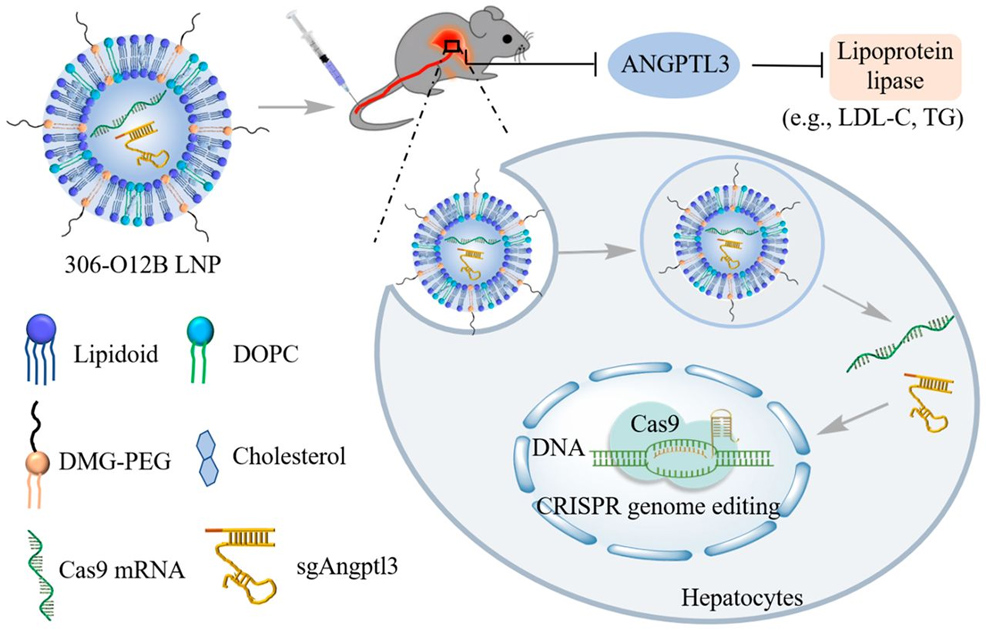
The genome editing technology CRISPR has emerged as a powerful new tool that can change the way disease is treated. The challenge when altering the genetics of our cells, however, is how to do it safely, effectively, and specifically targeted to the gene, tissue and organ that needs treatment. This week, scientists from Tufts University and the Broad Institute of Harvard and MIT report the development of nanoparticles comprised of lipids – fat molecules – that can package and deliver gene editing machinery specifically to the liver. In their study, published in the Proceedings of the National Academy of Sciences, they have shown how the lipid nanoparticles (LNPs) can efficiently deliver the CRISPR machinery into the livers of mice, resulting in specific genome editing and the reduction of blood cholesterol levels by as much as 57% – a reduction that can last for at least several months with just one shot. High cholesterol affects nearly 30 million Americans, according to the Centers for Disease Control and Prevention. The condition is complex and can originate from multiple genes as well as nutritional and lifestyle choices, so is not easy to treat. The Tufts and Broad researchers, however, have modified a single gene that could provide a protective effect against elevated cholesterol if shut down by gene editing.
The gene that the researchers focused on codes for the angiopoietin-like 3 enzyme (Angptl3). That enzyme restricts the activity of other enzymes – lipases – which help to break down cholesterol. If researchers can knock out the Angptl3 gene, they can let the lipases do their work and reduce the levels of cholesterol in the blood. It turns out that some lucky people have a natural mutation in their Angptl3 gene, resulting in consistently low levels of triglycerides and low-density lipoprotein (LDL) cholesterol, commonly called "bad" cholesterol, in their bloodstream without any known clinical downsides. "If we can replicate that condition by knocking out the angptl3 gene in others, we have a good chance of having a safe and long-term solution to high cholesterol," said Qiaobing Xu, associate professor of biomedical engineering at Tufts and corresponding author of the study. "We just have to make sure we deliver the gene editing package specifically to the liver, so as not to create unwanted side effects." Xu's team achieved precisely that in mouse models. After a single injection of nanoparticles packed with mRNA coding for CRISPR-Cas9 and a single-guide RNA targeting Angptl3, they observed a profound reduction in LDL cholesterol by as much as 57% and triglyceride levels by 29%, both of which remained at those lowered levels for at least 100 days. The researchers speculate that the effect may last much longer than that, perhaps limited only by the slow turnover of cells in the liver, which can occur over a period of about a year. The reduction of cholesterol and triglycerides is dose-dependent, so their levels could be adjusted by injecting fewer or more LNPs in the single shot, the researchers said.
By comparison, an existing, FDA-approved version of CRISPR mRNA-loaded LNPs could only reduce LDL cholesterol by at most 15.7% and triglycerides by 16.3% when tested in mice, according to the researchers. The key to making a better LNP lay in customising the components – the molecules that come together to form bubbles around the mRNA. The LNPs are composed of long chain lipids with a charged or polar head that is attracted to water, a carbon chain tail that points toward the middle of the bubble containing the payload, and a chemical linker between them. Also present are polyethylene glycol, and even some cholesterol – which has a normal role in lipid membranes to make them less leaky – to hold their contents better. The nature and relative ratio of these components had profound effects on the delivery of mRNA into the liver, so the researchers tested LNPs with many combinations of heads, tails, linkers and ratios among all components for their ability to target liver cells. Because the in vitro potency of an LNP formulation rarely reflects its in vivo performance, they directly evaluated the delivery specificity and efficacy in mice that have a "reporter" gene that lights up red when genome editing occurs. Ultimately, they found a CRISPR mRNA-loaded LNP that lit up just the liver in mice, showing that it could specifically and efficiently deliver gene-editing tools into the liver to do their work. "CRISPR is one of the most powerful therapeutic tools for the treatment of diseases with a genetic etiology," explained Min Qiu, post-doctoral researcher in Xu's lab at Tufts. "We have recently seen the first human clinical trial for CRISPR therapy enabled by LNP delivery to be administered systemically to edit genes inside the human body. Our LNP platform developed here holds great potential for clinical translation." "We envision that with this LNP platform in hand, we could now make CRISPR a practical and safe approach to treat a broad spectrum of liver diseases or disorders," said Zachary Glass, graduate student in the Xu lab.
Comments »
If you enjoyed this article, please consider sharing it:
|