30th June 2023 New drug provides weight loss of 24% In a significant advance for the treatment of obesity, biotech giant Eli Lilly has announced the results from a trial of retatrutide, which produced a staggering 24.2% weight loss in patients.
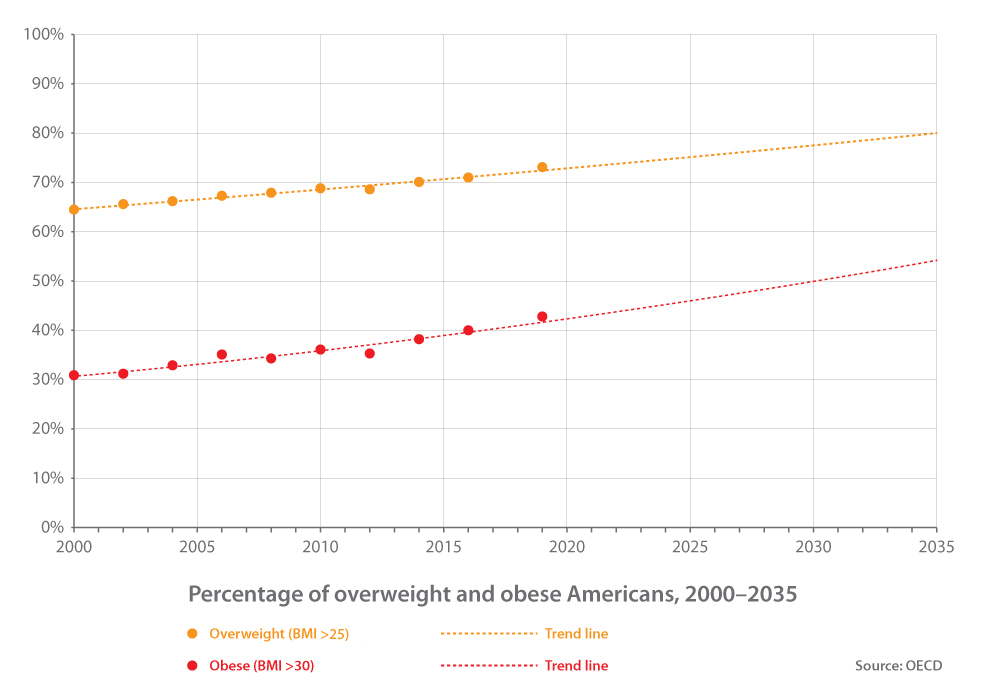
Obesity, defined as a body mass index (BMI) of 30 or higher, is a major health issue that has increased significantly over the years. As of today, it affects over 42% of adults in the United States, with 20% of children and adolescents also affected. Worldwide, more than 1 billion people are dealing with the condition, including 650 million adults, 340 million adolescents, and 39 million children. The health impacts of obesity are severe, with four million people dying each year as a result of obesity-related complications. These include various chronic medical conditions like type 2 diabetes, heart disease, and some forms of cancer. In the United States, adults with obesity spend an average of $1,861 more a year on medical costs. Those with severe obesity (a BMI of 40 or higher) spend an average of $3,097 more a year than non-obese people. Total medical costs related to obesity are estimated at $260.6 billion each year. The worldwide obesity rate has more than doubled since 1980, and the trend continues to worsen. One study has predicted that the percentage of obese people in the world is likely to increase from 14% to 22% by 2045. The United States, among the worst affected countries, will see its obesity rate increase from 42% to 50% by 2030. When including those classed as overweight (BMI >25), more than 80% of the country is projected to have an unhealthy weight by 2035.
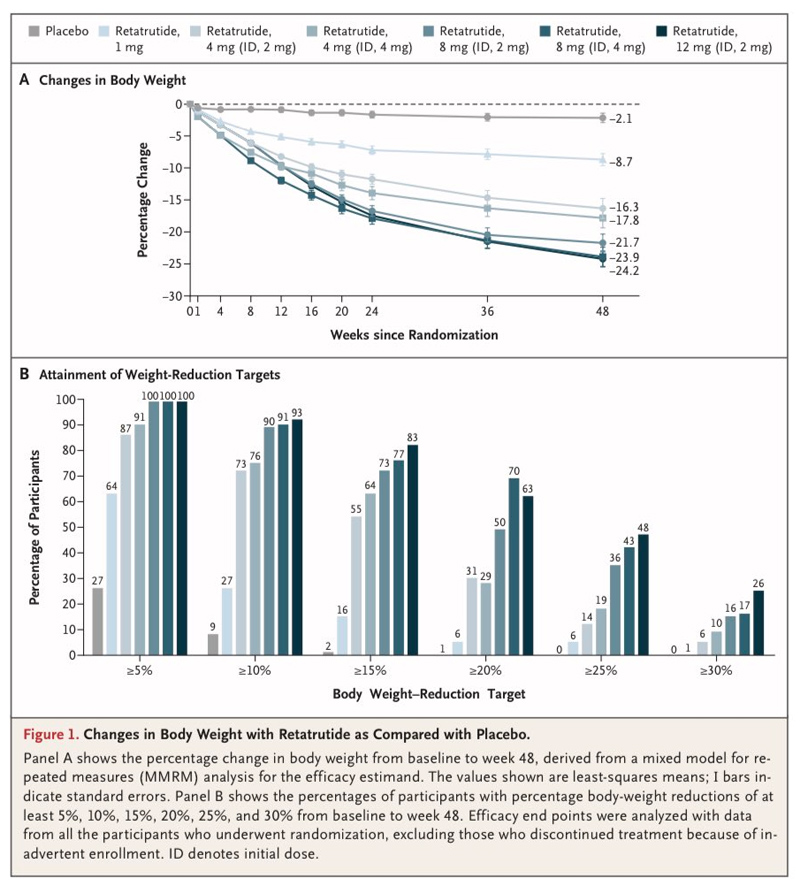
However, these alarming trends could be slowed – or perhaps one day reversed – if new drugs and other treatments become available to help those with obesity lose weight more easily. For example, a gene therapy developed in 2019 succeeded in "turning off" a fat storage gene in mice, resulting in a 20% weight loss and improved insulin resistance. In that same year, the standard FDA-approved treatment offered a weight loss of only 5% to patients. But more recently, human clinical trials have been showing greater success and are now beginning to catch up with animal models. A study of semaglutide in 2021 found that a weekly 2.4 mg dose taken for 68 weeks produced an average weight loss of 14.9% in human volunteers. A study published last year, in which people took the diabetes drug tirzepatide (sold under the brand name Mounjaro), found that a majority of those on the highest dose had a reduction in body weight of 20% or more after 72 weeks. The FDA approved Mounjaro for diabetes in May 2022 and is expected to approve it for weight loss in November 2023. Now, an even greater percentage weight loss has been achieved, with a drug called retatrutide. Eli Lilly and Company has just published the results from a recent trial in The New England Journal of Medicine. The company also presented its findings at the recent American Diabetes Association conference held in San Diego. The Phase 2 trial of retatrutide involved 338 adults, 52% of whom were men, each with a minimum BMI of 27. Participants on the highest dose achieved a mean weight reduction of 24.2%, translating to an average absolute weight reduction of about 58 lbs (26.3 kg) over 11 months of the study. Given that participants had not yet reached a weight plateau at the time the study ended, full efficacy could have been even higher if allowed to continue for longer.
In addition to losing weight, study participants experienced improvements in cardiometabolic measures including systolic and diastolic blood pressure, triglycerides, LDL-cholesterol, total cholesterol, and glucose and insulin levels. Gastrointestinal side effects were the most commonly reported adverse events, were generally mild-to-moderate, and usually occurred during the dose escalation period. Following these highly promising results, Eli Lilly and Company has started enrolling participants in a much larger Phase 3 clinical trial, which is expected to conclude in December 2025. If successful, it would then need to go through the FDA's rigorous review process, after which the general public could begin receiving prescriptions for retatrutide. "We believe that combining glucagon receptor agonism with GIP and GLP-1 receptor agonism may be one of the reasons retatrutide showed this level of weight reduction," explained Dan Skovronsky, PhD, Chief Scientific and Medical Officer at Eli Lilly. "These Phase 2 data have given us confidence to further explore the potential of retatrutide in Phase 3 trials that will look beyond weight reduction and focus on treating obesity and its complications comprehensively." "Obesity is a treatable chronic disease with a complex underlying biology. We are now in the midst of a rapidly expanding therapeutic landscape of potential highly effective treatment options for individuals with obesity," said Ania Jastreboff, PhD, study co-author and Director of the Yale Obesity Research Center.
Comments »
If you enjoyed this article, please consider sharing it:
|