15th November 2023 Gene therapy cuts cholesterol by up to 55% A new gene-editing therapy dramatically reduced bad cholesterol (LDL-C) in people at high risk of early heart attack, according to interim results from Verve Therapeutics in Boston.
Unhealthy vs. healthy blood vessel. Credit: tigatelu
Biotech startup Verve Therapeutics is pioneering a novel approach to cardiovascular disease with single-course gene editing. The company, whose investors include Google Ventures, has just announced its first human data for in vivo base editing from a Phase 1b clinical trial of VERVE-101. VERVE-101 is designed to treat heterozygous familial hypercholesterolemia (HeFH), a life-threatening inherited disease that is characterised by elevated levels of low‑density lipoprotein (LDL, or "bad" cholesterol) in the blood, alongside atherosclerotic cardiovascular disease (ASCVD). Cardiovascular disease (CVD) is the number one cause of death globally, and hundreds of millions of people are living with ASCVD, a subset of CVD. There are approximately 800,000 heart attacks each year in the U.S. alone, with a person dying every 34 seconds from CVD. VERVE-101 is a single-course treatment that works by permanently turning off the PCSK9 gene in the liver, to durably lower the blood's LDL. This Phase 1b trial involved nine participants with an average age of 54, and with average LDL cholesterol level of 201 mg/dL (5.2 mmol/L). For comparison, a healthy level is considered to be less than 116 mg/dL (3 mmol/L).
PCSK9. Credit: Emw, CC BY-SA 3.0, via Wikimedia Commons
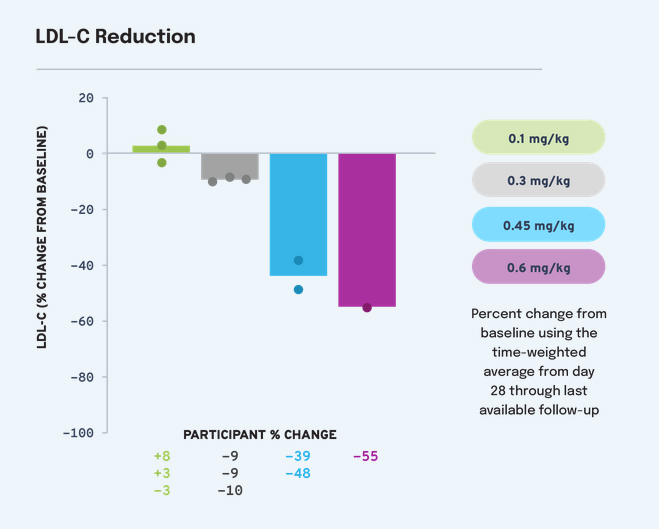
The nine people, split into four groups, received doses ranging from 0.1 mg/kg to 0.6 mg/kg. For the highest dose, the researchers found a reduction in LDL cholesterol levels of 55%, falling well within the healthy range. This remained effective for six months and is ongoing. In a previous study involving monkeys, VERVE-101 lowered PCSK9 levels by 67%-83% and LDL cholesterol by 49%-69%, depending on the dose. The effect on these animals continues to be observed after 2.5 years. "We were thrilled to see that the previous testing we had done of VERVE-101 in animal models translated faithfully to these findings in humans," said the senior study author Andrew Bellinger, PhD, Chief Scientific Officer at Verve Therapeutics.
"We are excited to have reached this milestone of positive first-in-human data supporting the significant potential for in vivo liver gene editing as a treatment for patients with HeFH," said Sekar Kathiresan, MD, co-founder and CEO. "VERVE-101 is the first in vivo base editor to be evaluated in the clinic. This milestone is only possible because of the incredible patients, families, and physicians who are participating in our study, and the highly talented team at Verve in their steadfast commitment to bringing VERVE-101 forward." "Our goal is to fundamentally disrupt the chronic care model for cardiovascular disease and provide a new single-course treatment option for patients," added Bellinger. "These data confirm our hypothesis that a single-course gene editing medicine has the potential to induce meaningful and durable reductions in LDL-C when administered at therapeutic doses." This first phase involved patients from the UK and New Zealand. Following the recent approval of an application to the U.S. Food and Drug Administration (FDA), Verve now plans to enrol and study patients in the United States. In 2024, the company will expand its cohort numbers and is also planning to initiate testing of a second treatment type called VERVE-102. This aims to inactivate the PCSK9 gene in a similar way to VERVE-101 but delivered using the company's proprietary GalNAc-LNP technology, designed to optimise recognition and binding to receptors. Phase 2 clinical trials of either VERVE-101 or VERVE-102 are expected in 2025. Each participant will also be placed into a long-term follow-up study for an additional 14 years, as required by the FDA for all participants in any human genome editing trials.
Comments »
If you enjoyed this article, please consider sharing it:
|