20th February 2026 Researchers discover how to "switch off" cancer genes for good Scientists have uncovered a mechanism that enables certain cancer genes to be permanently silenced, potentially allowing shorter and more effective treatments with fewer side effects. The findings could reshape the future of epigenetic therapy for aggressive blood cancers.
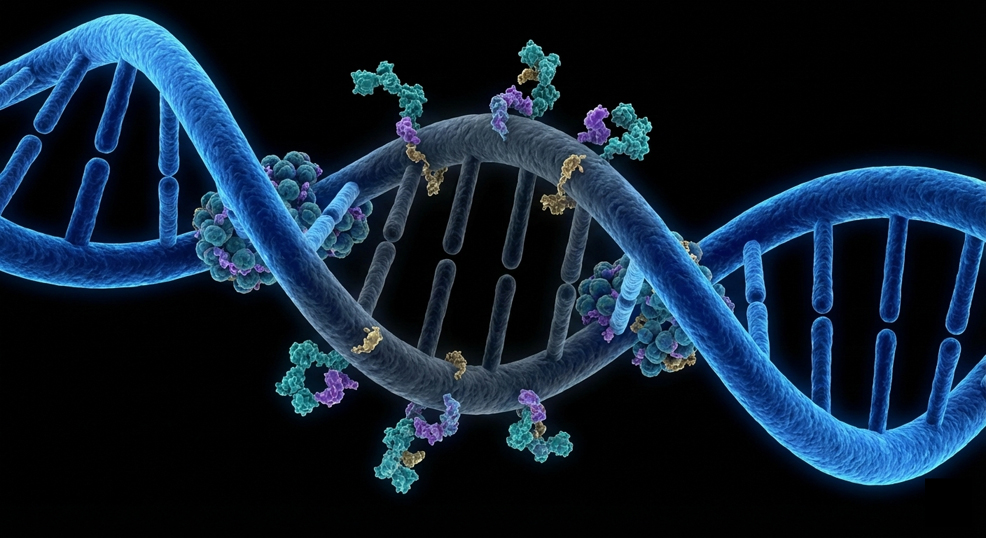
Monash University researchers, in collaboration with Harvard University, have discovered how to permanently "switch off" cancer-causing genes, revealing a new approach to cancer treatment. The breakthrough, published in the prestigious Nature Cell Biology, could result in shorter treatment periods and dramatically reduce the debilitating side effects that often come with cancer treatment. The research focuses on so-called epigenetic therapy, where patients take a drug to alter how their genes are switched on or off, resetting the harmful changes in gene activity caused by cancer mutations. This includes aggressive forms of leukaemia driven by a genetic error that hijacks the cell's machinery, keeping cancer-promoting genes constantly switched on. Drugs that act on this process are already in development, but until now, scientists did not fully understand how they work. The team showed that, in certain types of leukaemia, a group of "gene control" proteins – including Menin and DOT1L – keeps dangerous genes switched on. DOT1L puts chemical tags on histones (pictured below), the spool-like proteins that DNA wraps around, creating a kind of molecular "memory" that tells a cell to keep those genes active. When Menin is blocked for long enough, this memory slowly fades, and the cell's own built-in silencing system can finally move in and switch those cancer-driving genes off. Omer Gilan, Senior Research Fellow at Monash University's School of Translational Medicine and the Australian Centre for Blood Diseases, led the research team behind the discovery that disrupting this balance can drive long-lasting shutdown of cancer-causing genes in leukaemia cells.
"We have potentially identified a new way to exploit cancer's weaknesses," said Dr Gilan. "But the most exciting part is that clinicians can harness our findings to improve response and reduce side effects for patients. Anyone who has watched someone they love go through cancer treatment will attest to how difficult it is – so making treatment easier to withstand and more effective is absolutely vital." Monash PhD candidate Daniel Neville, lead author on the Nature Cell Biology paper, said the improvement leverages the "memory" provided by the epigenetic protein DOT1L, found in cancer cells. "The drugs we use to target Menin erase the memory provided by DOT1L, and continue killing the cancer cells, even after the treatment has stopped," he said. "We hope that by reducing the treatment period, patients may tolerate higher doses or be eligible for additional therapies to improve outcomes. This is a big step forward for epigenetic therapy, and one we hope will change how cancer is treated more generally." The team's discovery is set to be tested in a human clinical trial run by Monash University and The Alfred in Melbourne, a major teaching and research hospital, later this year. Associate Professor Shaun Fleming, head of the myeloid disease program at The Alfred and a researcher at Monash's Australian Centre for Blood Diseases, says this is an exciting step forward for leukaemia treatment: "As we continue clinical trials of Menin inhibitors, and particularly moving into combination studies, understanding better how these new therapies work may allow us to utilise them more effectively and with a greater degree of safety in future."
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||