20th July 2013 'Impossible' material made with record-breaking surface area and water adsorption properties A novel material with record-breaking surface area and water adsorption abilities has been synthesised by researchers from Uppsala University, Sweden.
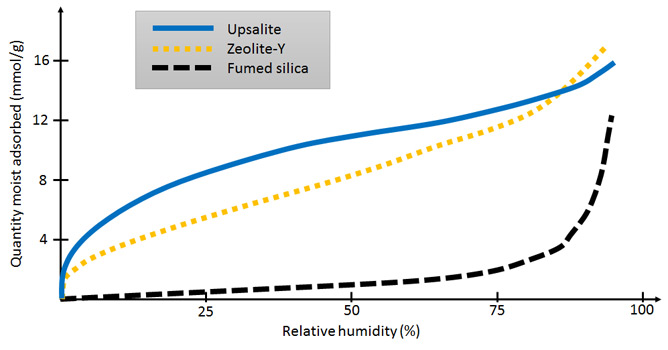
The new material – based on magnesium carbonate – has been given the name "Upsalite". It is filled with empty pores all less than 10 nanometres in size. This provides a total surface area of more than 800 square metres per gram: the largest ever achieved for an alkali earth metal carbonate. As a result, Upsalite will earn a place in the exclusive class of porous, high surface area materials which includes carbon nanotubes, metal organic frameworks, mesoporous silica and zeolites. The unique pore structure, seen in the photo above, enables it to absorb more water at low relative humidities than the best materials presently available. It could reduce the amount of energy needed for moisture control and filtering in the electronics and drug formulation industries, for example, as well as in warehouse environments. It could also be used in the collection of toxic waste, chemical/oil spills, odour control and sanitation after fire, cosmetics, ink jet paper and a new generation of biomaterials. Furthermore, it has a very low manufacturing cost.
"In contrast to what has been claimed for more than 100 years in scientific literature, we have found that amorphous magnesium carbonate can be made in a very simple, low-temperature process," says Johan Goméz de la Torre, researcher at the Nanotechnology and Functional Materials Division. "One Thursday afternoon, we slightly changed the synthesis parameters of the earlier employed unsuccessful attempts, and by mistake left the material in the reaction chamber over the weekend. Back at work on Monday morning we discovered that a rigid gel had formed and after drying this gel we started to get excited."
A year of detailed materials analysis and fine tuning of the experiment followed. One of the researchers got to take advantage of his Russian skill, since some of the chemistry details necessary for understanding the reaction mechanism were only available in an old Russian PhD thesis. "Having gone through a number of state-of-the-art materials characterisation techniques, it became clear that we had indeed synthesised the material that previously had been claimed impossible to make," says Prof. Maria Strømme, Head of the Nanotechnology and Functional Materials Division. The discovery was published this week in PLOS ONE. It will be commercialised through the University spin-out company Disruptive Materials, formed by the researchers together with the holding company of Uppsala University.
Comments »
|
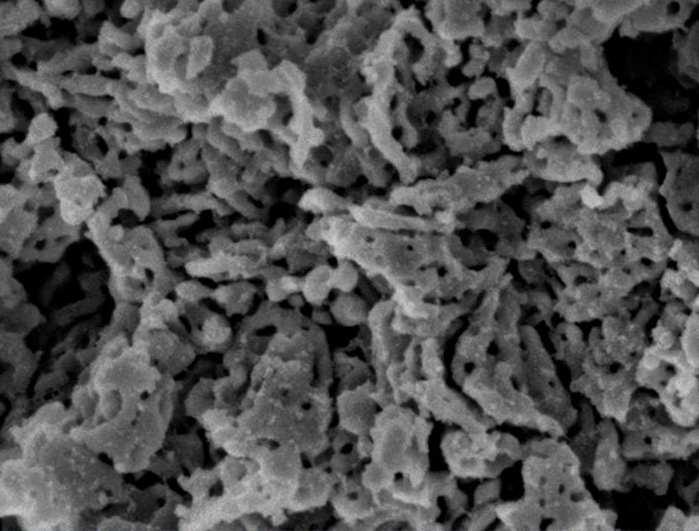 Electron microscopy images of Upsalite. Credit: Forsgren J, Frykstrand S, Grandfield K, Mihranyan A, Strømme M (2013)
Electron microscopy images of Upsalite. Credit: Forsgren J, Frykstrand S, Grandfield K, Mihranyan A, Strømme M (2013)