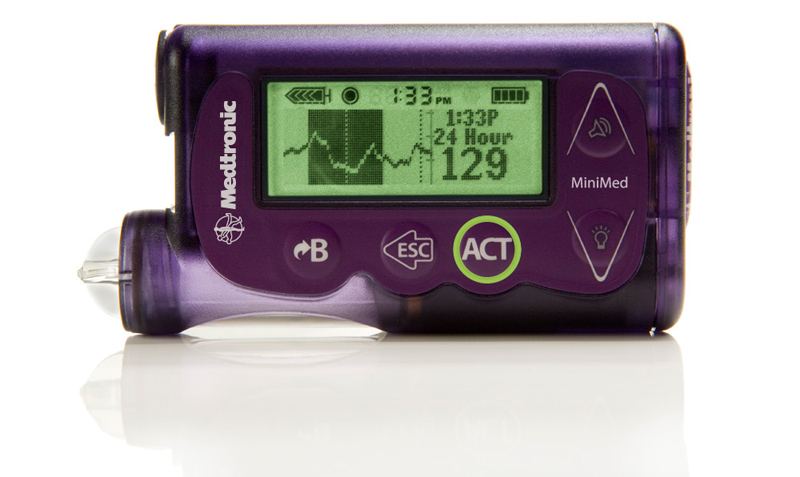
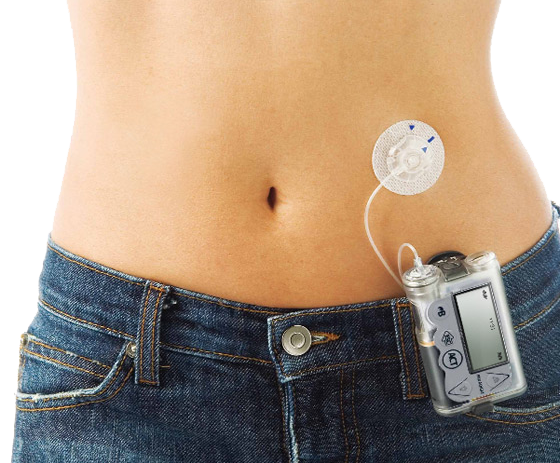
5th October 2013 First artificial pancreas approved by the FDA Medtronic, the world's largest medical technology company, has been granted approval by the FDA for its MiniMed 530G with Enlite – a breakthrough, first-generation artificial pancreas for diabetes sufferers.
Medtronic's system is the first in the U.S. that can automatically stop insulin delivery when sensor glucose values reach a preset level and when the patient doesn't respond to the Threshold Suspend alarm. The MiniMed 530G incorporates the new Enlite sensor, Medtronic's most accurate and comfortable continuous glucose sensor, which has a 31 percent improvement in overall accuracy over previous sensors. “We’re excited to bring yet another important ‘first’ to the United States. The MiniMed 530G with Enlite can help people gain better control of their diabetes versus multiple daily injections,” said Katie Szyman, president of the Diabetes business at Medtronic. “We are committed to advancing closed loop algorithms, continuous glucose monitoring and insulin delivery technologies to bring new artificial pancreas systems to market.”
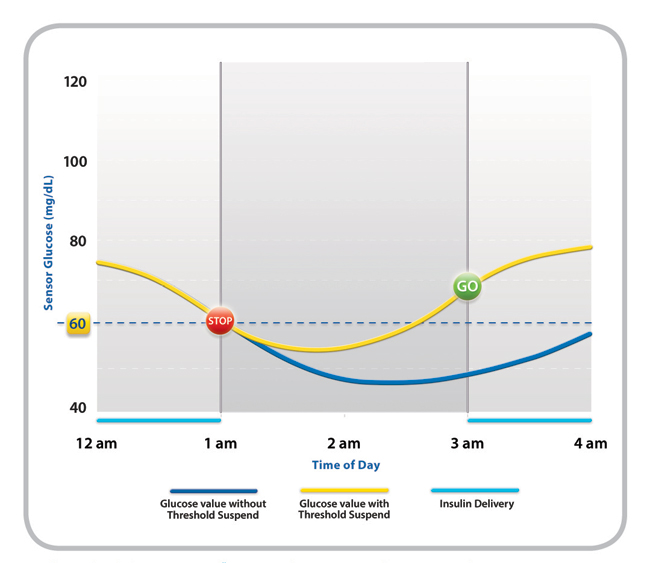
In addition to improved comfort and more accurate Continuous Glucose Monitoring (CGM), the Enlite sensor detects up to 93 percent of hypoglycemia episodes when predictive and threshold alerts are on. It is nearly 70 percent smaller than the previous Medtronic sensor and provides a simpler insertion process with a hidden-introducer needle. The MiniMed 530G is the first system approved under the new product classification, “OZO: Artificial Pancreas Device System, Threshold Suspend,” created by the U.S. Food and Drug Administration. Threshold Suspend automation automatically stops the delivery of insulin if glucose levels reach a threshold, which can be set by a healthcare provider between 60-90 mg/dL. Once the threshold is met, the MiniMed 530G will first alert the wearer with an alarm. If the individual is sleeping, unconscious or otherwise unable to react, the device will suspend all insulin delivery for two hours. Insulin delivery can be resumed at any time. The MiniMed 530G system was approved for use by people with diabetes aged 16 and older. Medtronic will now conduct a post-approval study and will engage in direct patient follow-up. The company intends to begin ramping up production immediately to prepare for launch of the device in the next several weeks.
Comments »
|