23rd January 2015 Long-term sequestration of carbon may be harder to achieve than previously thought Long-term carbon sequestration is viewed as a way of mitigating climate change. It may be harder to achieve than previously thought, however, due to problems converting the gas to a solid state after injection underground, MIT reports.
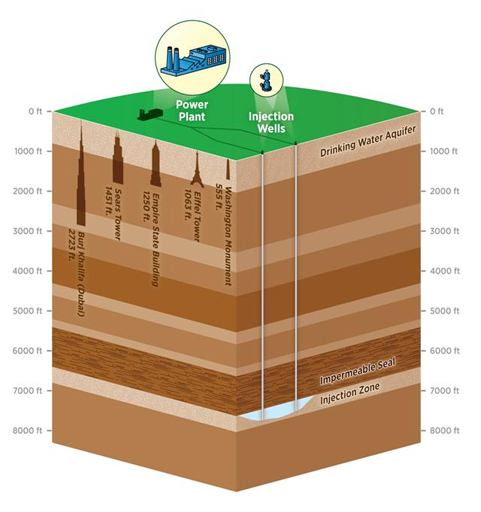
Carbon sequestration promises to address human-made greenhouse-gas emissions by capturing carbon dioxide from the atmosphere and injecting it deep below the Earth’s surface, where it would permanently solidify into rock. The U.S. Environmental Protection Agency estimates that current sequestration technologies may eliminate up to 90 percent of carbon dioxide emissions from coal-fired power plants. While such technologies may successfully remove greenhouse gases from the atmosphere, keeping them locked underground is another matter entirely. Researchers in the Department of Earth, Atmospheric and Planetary Sciences at MIT have found that once injected into the ground, less carbon dioxide is converted to rock than previously imagined. The team studied the chemical reactions between carbon dioxide and its surroundings once the gas is injected into the Earth – finding that as carbon dioxide works its way underground, only a small fraction turns to rock. The remaining gas stays in a more tenuous form. “If it turns into rock, it’s stable and will remain there permanently,” says Yossi Cohen, a postdoctoral research associate. “However, if it stays in its gaseous or liquid phase, it remains mobile and it can possibly return back to the atmosphere.” Current techniques aim to inject carbon dioxide into the subsurface some 7,000 feet below ground – a depth equivalent to five Empire State Buildings stacked end-to-end. At such depths, carbon dioxide is stored in deep-saline aquifers: large pockets of brine that can chemically react to solidify the carbon dioxide gas.
Cohen and Daniel Rothman, a professor of geophysics, sought to model the chemical reactions that occur after carbon dioxide is injected into a briny, rocky environment. When carbon dioxide is pumped into the ground, it rushes into open pockets within rock, displacing any existing fluid, such as brine. What remains are bubbles of carbon dioxide, along with carbon dioxide dissolved in water. The dissolved carbon dioxide takes the form of bicarbonate and carbonic acid, which create an acidic environment. To precipitate, or solidify into rock, carbon dioxide requires a basic environment, such as brine. The researchers modelled the chemical reactions between two main regions: • An acidic, low-pH region, with a high concentration of carbon dioxide • A higher-pH region filled with brine, or salty water As each carbonate species reacts differently when diffusing or flowing through water, the team characterised each reaction, then worked each one into a reactive diffusion model – a simulation of chemical reactions as carbon dioxide flows through a briny, rocky environment. When the team analysed the chemical reactions between regions rich in carbon dioxide and regions of brine, they found that the carbon dioxide solidifies – but only at the interface. The reaction essentially creates a solid wall at the point where carbon dioxide meets brine, keeping the bulk of the gas from reacting with the brine. “This can basically close the channel, and no more material can move farther into the brine, because as soon as it touches the brine, it will become solid,” Cohen says. “The expectation was that most of the carbon dioxide would become solid mineral. Our work suggests that significantly less will precipitate.” Cohen and Rothman point out that their theoretical predictions require experimental study to determine the magnitude of this effect. “Experiments would help determine the kind of rock that would minimise this clogging phenomenon,” Cohen says. “There are many factors, such as the porosity and connectivity between pores in rocks, that will determine if and when carbon dioxide mineralises. Our study reveals new features of this problem that may help identify the optimal geologic formations for long-term sequestration.”
Comments »
|