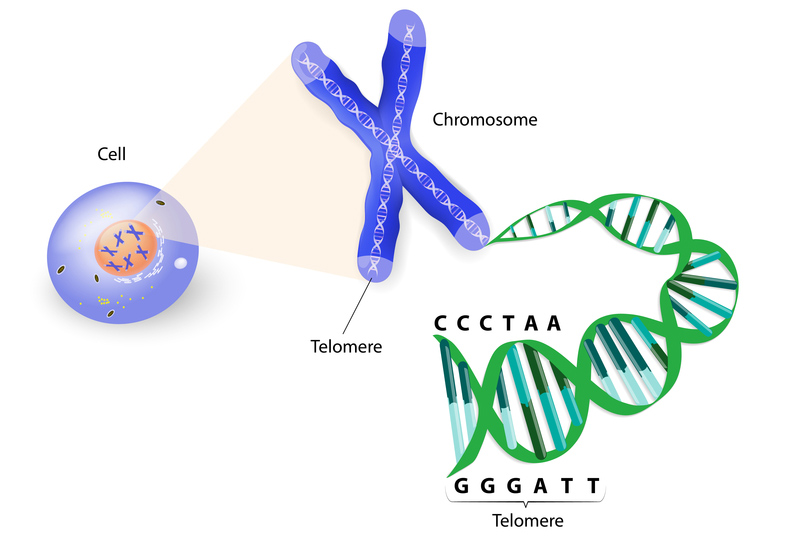
25th January 2015 Telomere extension in cultured human cells could lead to anti-aging therapies Researchers at Stanford University have demonstrated a fast and reliable method of extending the length of telomeres – the protective caps at the end of chromosomes that play a key role in aging.
As illustrated above, telomeres are regions of DNA at the ends of chromosomes. Like the plastic tips at the end of shoelaces, they protect chromosomes from unravelling and deteriorating, or mixing with other chromosomes. Over time, however, telomeres will begin to erode and shorten. When telomeres become critically short, the cell enters an inactive state, stops dividing and dies. More and more cells reacting in this way causes tissue degeneration, which gradually results in aging and disease. A young human starts with telomeres around 8,000-10,000 nucleotides long, with each cell division reducing this length, so a person in their 80s will average 4,000-6,000 nucleotides. Researchers at Stanford University School of Medicine, in a study published this week by the FASEB Journal, have found a way to extend the length of human telomeres by up to 900 nucleotides – equivalent to over 10 years of additional lifespan. This was achieved with human muscle and skin cells in a Petri dish using modified messenger RNA (mRNA) containing TERT, a vital part of the telomerase complex. Telomerase is an enzyme that occurs naturally and is known to prevent the shortening of telomeres. It is common in stem cells, but most other cell types have very low levels. The new technique developed at Stanford was designed in a clever way that managed to optimise the available treatment time – maximising the effects of TERT by preventing an immune response being triggered in the cell, while minimising the danger of cancer that might result from TERT staying too long and causing too many divisions. A balance was achieved whereby TERT was able to remain temporarily for about 48 hours, long enough to increase telomere lengths by 10%, before dissipating harmlessly. During this time, cells divided many more times in the culture dish than did untreated cells: about 28 more times for the skin cells, and about three more times for the muscle cells. "We were surprised and pleased that modified TERT mRNA worked, because TERT is highly regulated and must bind to another component of telomerase," says co-author John Ramunas, PhD, in a press release. "Previous attempts to deliver mRNA-encoding TERT caused an immune response against telomerase, which could be deleterious. In contrast, our technique is nonimmunogenic. Existing transient methods of extending telomeres act slowly, whereas our method acts over just a few days to reverse telomere shortening that occurs over more than a decade of normal aging. This suggests that a treatment using our method could be brief and infrequent." "This new approach paves the way toward preventing or treating diseases of aging," says Helen Blau, PhD, a professor of microbiology and immunology at Stanford. "There are also highly debilitating genetic diseases associated with telomere shortening that could benefit from such a potential treatment. "We're working to understand more about the differences among cell types, and how we can overcome those differences to allow this approach to be more universally useful. One day, it may be possible to target muscle stem cells in a patient with Duchenne muscular dystrophy, for example, to extend their telomeres. There are also implications for treating conditions of aging, such as diabetes and heart disease. This has really opened the doors to consider all types of potential uses of this therapy."
Comments »
|