9th May 2019 Phage therapy treats patient with drug-resistant bacterial infection A British teenager is reported to be the first patient to receive a genetically modified (GM) phage therapy to treat a drug-resistant infection. 15-year-old Isabelle Holdaway had come to London's Great Ormond Street Hospital for a double lung transplant. Her lungs were struggling to reach even a third of their normal function. Holdaway had cystic fibrosis, a genetic disease that clogs lungs with mucus and causes persistent infections. For eight years, she had taken antibiotics to control two stubborn bacterial strains. Weeks after the transplant, doctors noticed redness at the site of her surgical wound and signs of liver infection. Then, they saw nodules – pockets of bacteria pushing up through the skin – on her arms, legs, and buttocks. The infection was spreading, and traditional antibiotics were no longer working. However, a new personalised treatment is now helping the girl heal. This uses genetically engineered bacteriophages, tiny spider-shaped viruses able to infect and kill bacteria. Over the next six months, nearly all of the girl's skin nodules disappeared, her surgical wound began closing, and her liver function improved. Scientists reported the results yesterday in Nature Medicine. This breakthrough is the first to demonstrate safe and effective use of engineered bacteriophages in a human patient, says Graham Hatfull, a Professor of Biotechnology at the University of Pittsburgh. Such treatments could help in countering drug-resistant bacteria, a growing problem that is predicted to become as deadly and widespread as cancer by 2050, unless new medicines are developed. "The idea is to use bacteriophages as antibiotics – as something we could use to kill bacteria that cause infection," says Hatfull.
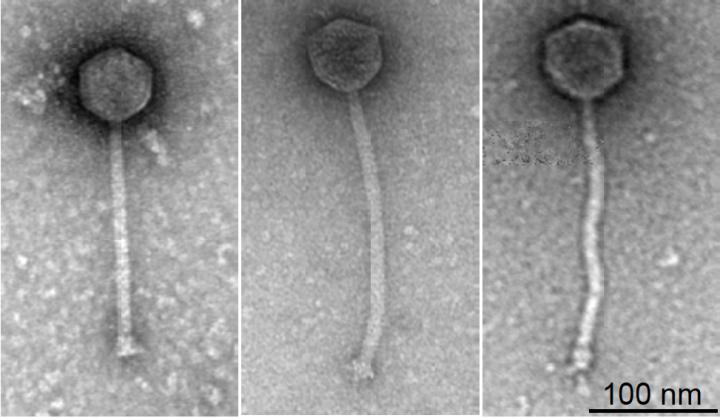
Professor Hatfull, a molecular geneticist, had spent over three decades amassing a colossal collection of bacteriophages, or phages, from the environment. Hatfull's colleague asked whether any of these phages could target the patients' strains of Mycobacterium. It was a fanciful idea, Hatfull says, and he was intrigued. His phage collection – the largest in the world – is contained in 15,000 vials and filled the shelves of two large freezers in his laboratory. They had been collected from thousands of different locations worldwide, largely by students. It is estimated that over a nonillion (that's a quadrillion times a quadrillion) phages exist in the Earth's atmosphere, soil and water. Hatfull leads a program called SEA-PHAGES, which offers students the opportunity to hunt for phages. Nearly 120 universities and colleges around the USA participated in the program last year. After testing samples, students can look in more detail under an electron microscope and then sequence the genome of any phages they have discovered, determine how well they infect and kill bacteria and see where they fit on the phage family tree. "I had a sense that this collection was enormously powerful for addressing all sorts of questions in biology," Hatfull says. "But we didn't think we'd ever get to a point of using these phages therapeutically." The idea of phage therapy has been around for nearly a century. But until recently, there wasn't much data about safety and efficacy. In April 2017, a team of researchers in California successfully used phages to treat a patient with a multidrug-resistant bacterium. That case, and the rise of antibiotic resistance, has led to growing interest in phages, Hatfull says. After receiving samples from the infected patient in London, his team searched their collection for phages that could potentially target the bacterial strain. From thousands of candidates, they narrowed it down to three: named Muddy, BPs and ZoeJ.
However, the study required some additional genetic tweaks. Muddy could infect the girl's bacteria – but ZoeJ and BPs weren't quite so efficient. So Hatfull modified these two phages, by removing a gene that allows them to reproduce harmlessly within a bacterial cell. Without the gene, phages continue to reproduce until they eventually burst from inside the cell, destroying it. They then combined the trio into a phage cocktail, purified it and tested it for safety. Doctors administered the cocktail to the patient via an IV twice daily, with a billion phages in every dose. After six weeks, a liver scan revealed that the infection had essentially disappeared. Today, only one or two of the girl's skin nodules remain. Hatfull has high hopes: the bacteria haven't shown any signs of developing resistance, and his team has prepped a fourth phage to add to the mix. "This program engages beginning students in real science," says David Asai, director of SEA-PHAGES. "Whatever they discover is new information. Now the phage collection has actually contributed to helping a patient." Finding the right phages for each patient is a big challenge, Hatfull says. One day, scientists may be able to create a phage cocktail that works more broadly to treat diseases. "We're sort of in uncharted territory," he says. But the basics of the young girl's case are pretty simple: "We purified the phages, we gave them to the patient, and the patient got better."
Comments »
If you enjoyed this article, please consider sharing it:
|