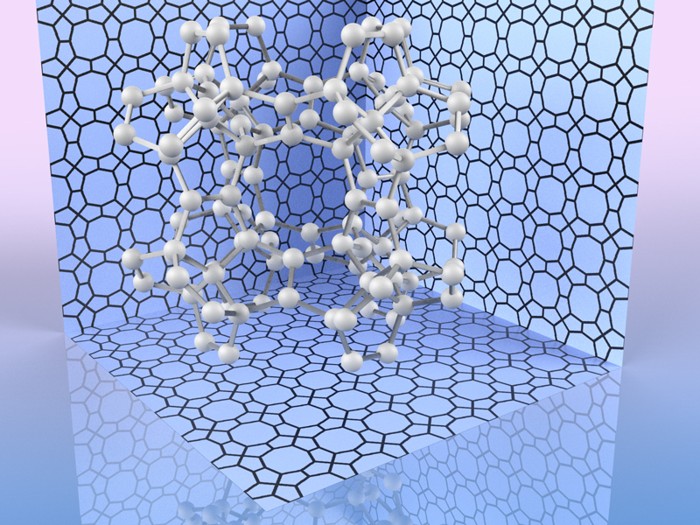
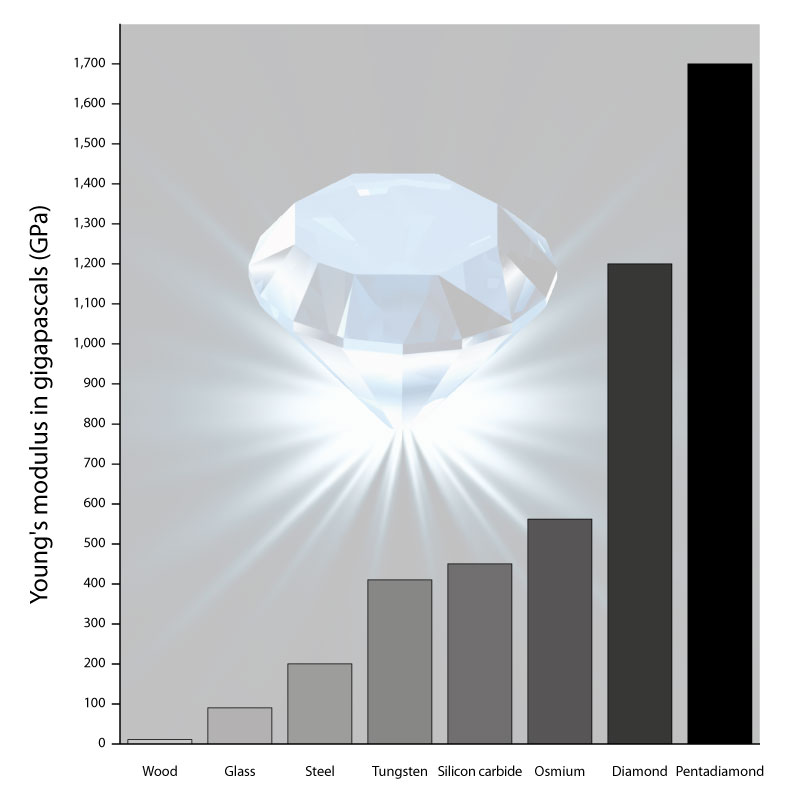
16th July 2020 Pentadiamond, a new addition to the carbon family Pentadiamond has been theorised by scientists in Japan. They calculate it has a Young's modulus of almost 1700 GPa, compared with 1200 GPa for conventional diamond.
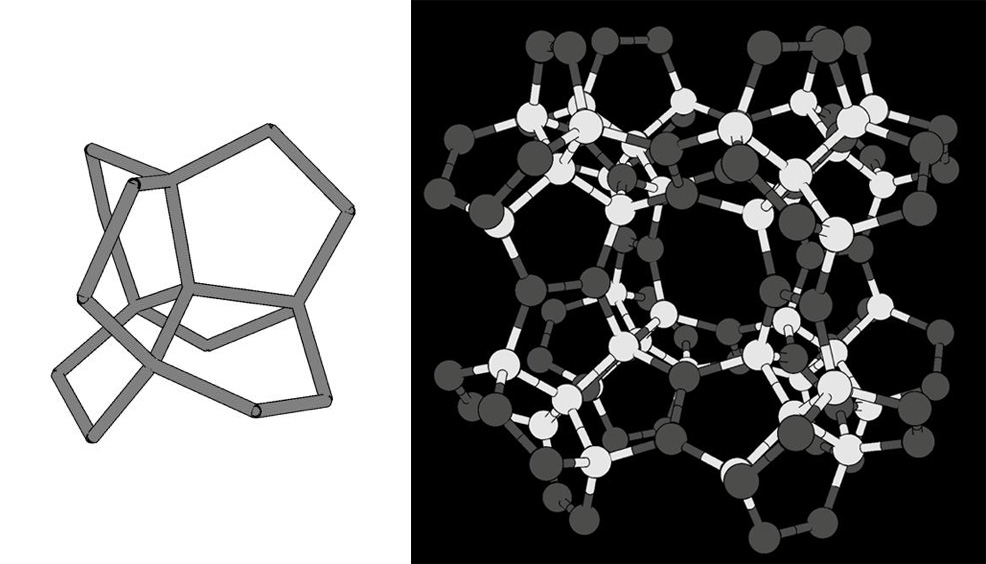
Researchers from the University of Tsukuba, Japan, have used computer calculations to design a new carbon-based material. This theoretical structure has been dubbed "pentadiamond" and is even harder than diamond, the hardest naturally occurring material. It may be useful in industrial cutting applications that rely on synthetic cutting diamonds, or tasks involving extremely high pressures. Diamonds, which are made entirely of carbon atoms arranged in a dense lattice, are famous for their unmatched hardness. However, carbon is known to form other stable configurations called allotropes. These include the familiar graphite in pencil lead, as well as new and emerging materials such as carbon nanotubes. The mechanical properties, including hardness, of an allotrope depend mostly on the way its atoms bond with each other. In conventional diamond, each atom forms a covalent bond with four neighbours. This arrangement is known as sp3 hybridisation. Nanotubes and some other materials form three bonds, which is called sp2 hybridisation. Now, researchers at the University of Tsukuba have explored what would happen if carbon atoms were arranged in a more complex structure with a mixture of sp3 and sp2 hybridisation. "Carbon allotropes with both sp2 and sp3 hybridised atoms have greater morphological diversity, due to the huge number of combinations and arrangements in networks," explains Yasumaru Fujii, first author of a scientific paper that appears in Physical Review Letters.
To calculate the most stable atomic configuration, as well as estimate its hardness, the team relied on a computational method called density functional theory (DFT). DFT has been successfully used throughout chemistry and solid-state physics to predict the structure and properties of materials. Keeping track of the quantum states of all the electrons in a sample, and their interactions, is usually an intractable task. Instead, DFT uses an approximation that focuses on the final density of electrons in space orbiting the atoms. This simplifies the calculation to make it suitable for computers, while still providing very precise results. Based on these calculations, the scientists found that the Young's modulus, a measure of hardness, for pentadiamond is predicted to be almost 1700 GPa – compared with about 1200 GPa for conventional diamond. "Not only is pentadiamond harder than conventional diamond, its density is much lower, equal to that of graphite," explains co-author Professor Mina Maruyama. "This work shows the power of designing materials ab initio. In addition to industrial cutting and drilling uses, pentadiamonds might be used in place of diamond anvil cells currently used in scientific research to recreate the extreme pressure inside planets," said Professor Susumu Okada, senior co-author.
Comments »
If you enjoyed this article, please consider sharing it:
|